
Margaret Patrick
Margaret Patrick joined Market Realist in September 2014 and has written close to 3,000 articles. She has covered the healthcare sector, which includes pharmaceutical and biotechnology companies, medical device companies, health insurance companies, and hospital companies. Currently, she is following the cannabis sector.
Prior to joining Market Realist, Margaret worked as equity and data analyst at MSCI for a year and as a financial research analyst at Deloitte for two years. She completed her MBA with finance specialization in 2011. She also passed all three CFA levels.
Besides writing on stocks, Margaret loves to read about nutrition, culture, and mythology. She's also fond of traveling to new places.
Disclosure: I am in full compliance with all ethics and other policies for Market Realist research analysts. I am not invested in securities that I cover on Market Realist.
More From Margaret Patrick
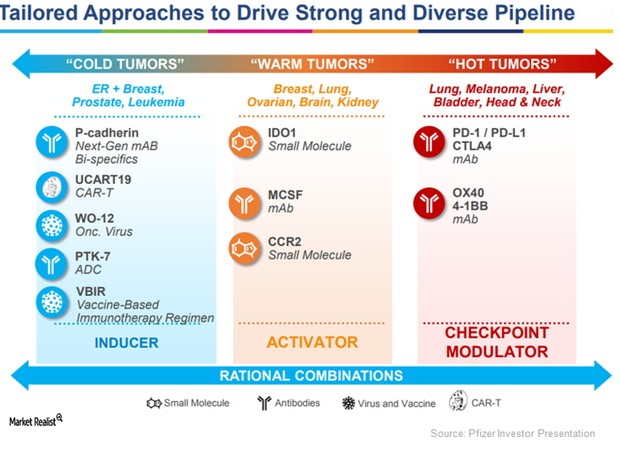
Pfizer Is Pursuing Oncology, Inflammation, and Immunology Research
On May 9, 2017, the US Food and Drug Administration (or FDA) approved Pfizer (PFE) and Merck’s Bavencio (avelumab) as a treatment option for patients suffering from locally advanced or metastatic urothelial carcinoma (or UC).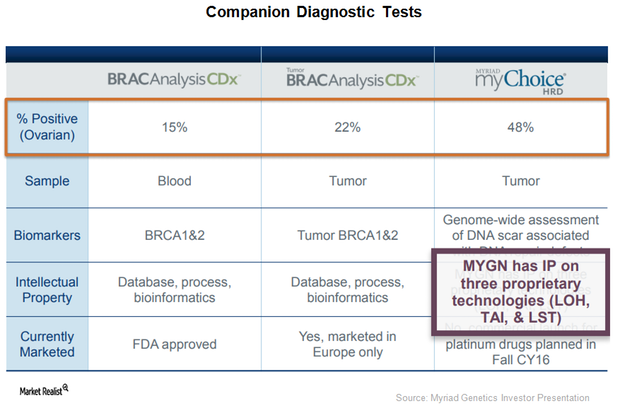
BRACAnalysis CDx Received FDA Approval for Ovarian Cancer Indication
On March 27, 2017, the FDA also approved BRACAnalysis CDX test as a complementary diagnostic test to be used with ovarian cancer maintenance therapy Tesaro’s (TSRO) Zejula (miraparib).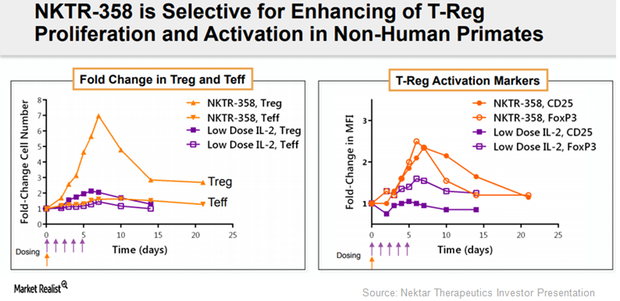
Nektar’s NKTR-358 Collaboration with Eli Lilly Boosted Revenues
Nektar Therapeutics (NKTR) recognized $128 million of the $150 million upfront payment from Eli Lilly (LLY) related to the development of investigational drug NKTR-358.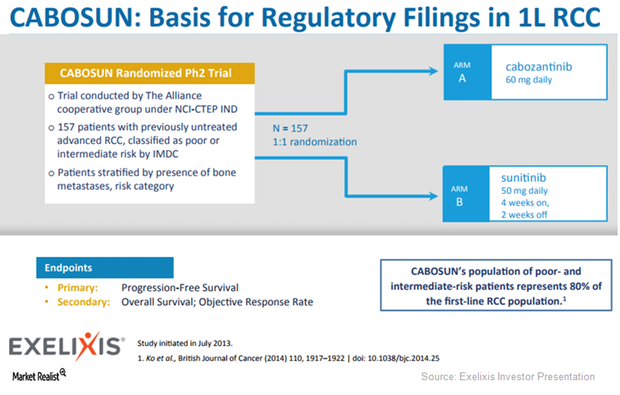
Behind Exelixis’s Cabometyx Strategy for 2018
Exelixis (EXEL) expects the FDA’s approval for Cabometyx for first-line RCC (renal cell carcinoma) to be a major revenue driver.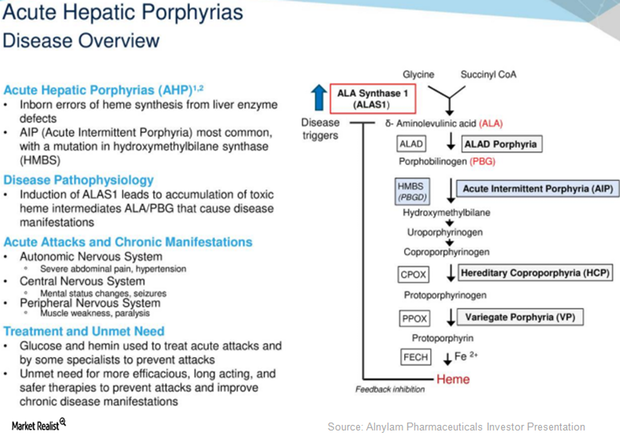
Givosiran May Become Major Growth Driver for Alnylam Pharmaceuticals
On September 7, 2017, Alnylam Pharmaceuticals (ALNY) announced that it had reached an agreement with the US Food and Drug Administration (or FDA) related to the design of the phase three program for investigational RNAi therapeutic Givosiran.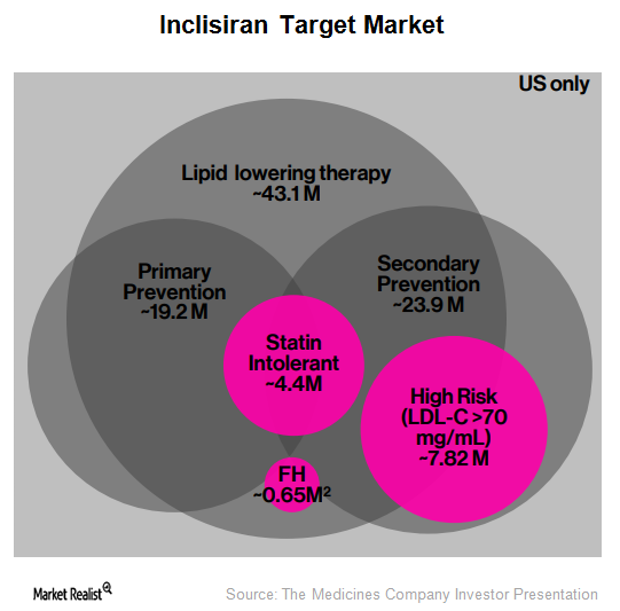
Inclisiran: Long-Term Growth Driver for The Medicines Company?
According to a Monte Carlo simulation performed at Harvard, it’s estimated that 5.0 million patients in the United States stand to benefit from PCSK9 inhibitor therapy.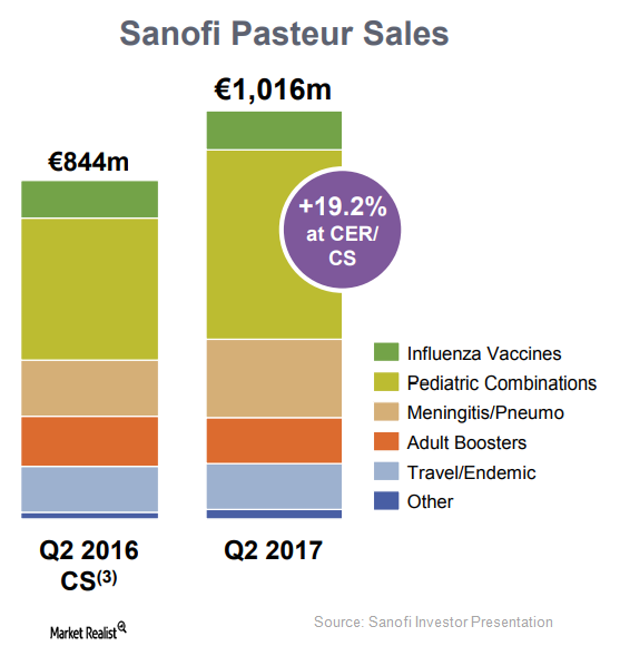
Vaccines Business Expected to Boost Sanofi’s Revenues in 2017
In 2Q17, Sanofi’s (SNY) vaccine business reported revenues of 1.0 billion euros, which is a YoY rise of 26.2% on a CER basis and 19.2% on a CER and CS basis.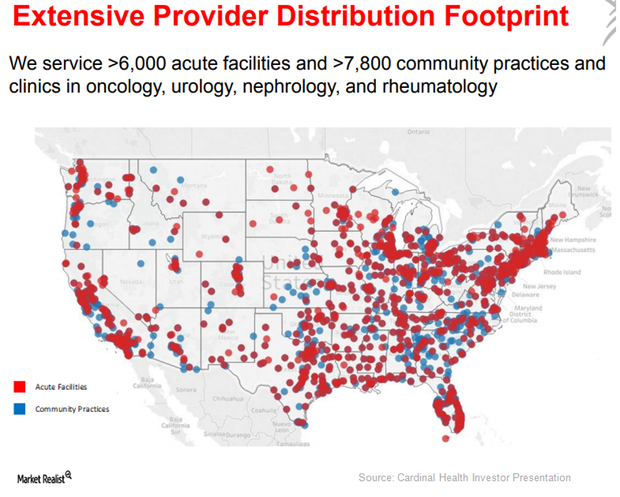
Robust Growth Expected for Cardinal Health’s Specialty Solutions
Cardinal Health’s (CAH) Specialty Solutions segment provides two types of services: upstream to pharmaceutical and biopharmaceutical manufacturers and downstream to healthcare providers.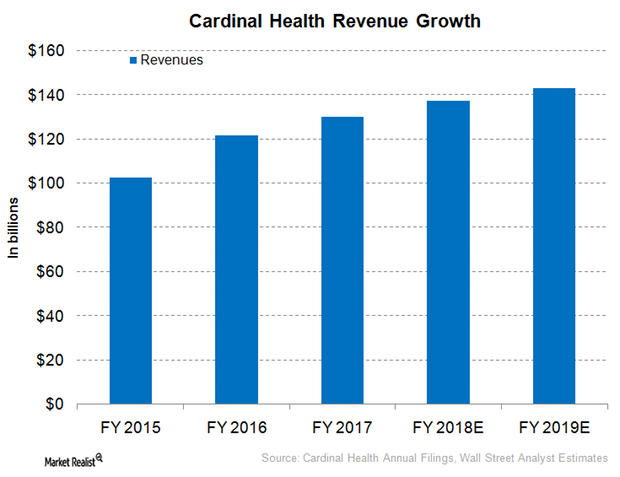
Cardinal Health Expected to Report Modest Revenue Growth
For fiscal 2018, Cardinal Health (CAH) has projected mid-single-digit revenue growth on a YoY basis, partially driven by the company’s high customer retention rates.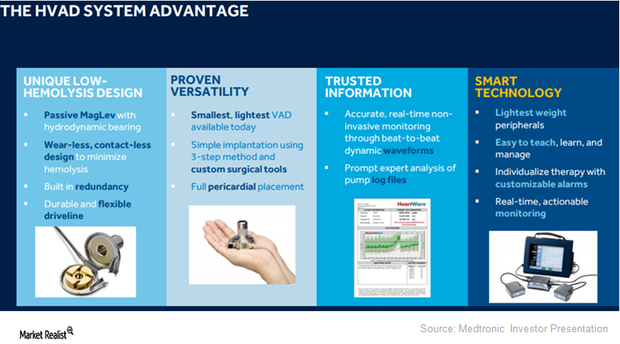
HVAD Expected to Expand Medtronic’s Presence in LVAD Segment
On September 27, 2017, the FDA approved Medtronic’s HVAD (HeartWare ventricular assist device) system as a destination therapy for advanced heart failure patients.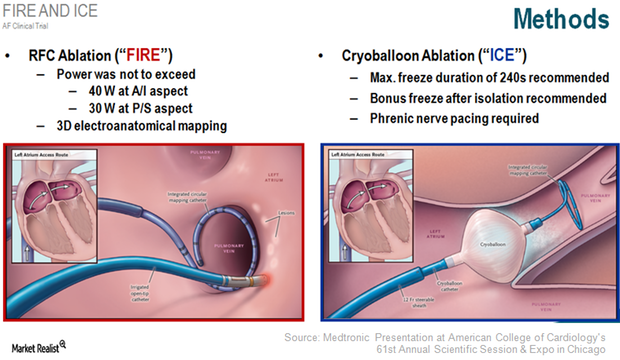
Atrial Fibrillation Ablation Could Be a Short-Term Growth Driver for MDT
More than 33 million patients suffer from AF, the most common form of heart arrhythmia. About 30% of these patients respond to antiarrhythmic drugs (or AAD).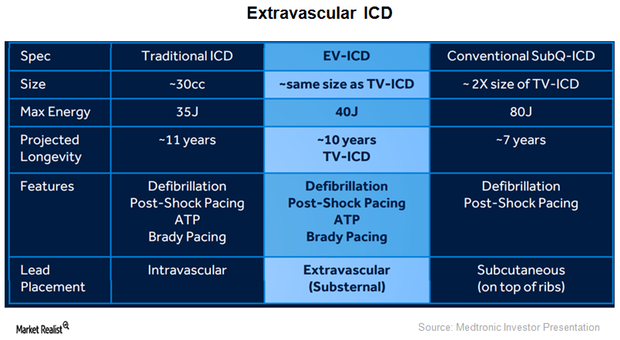
Medtronic Expands Capabilities of Implantable Cardioverter Defibrillators
On May 2, 2016, the FDA approved Medtronic’s (MDT) Visia AF and Visia AF MRI Surescan. These devices are single-chamber implantable cardioverter defibrillators (or ICDs) capable of detecting asymptomatic and undiagnosed atrial fibrillation.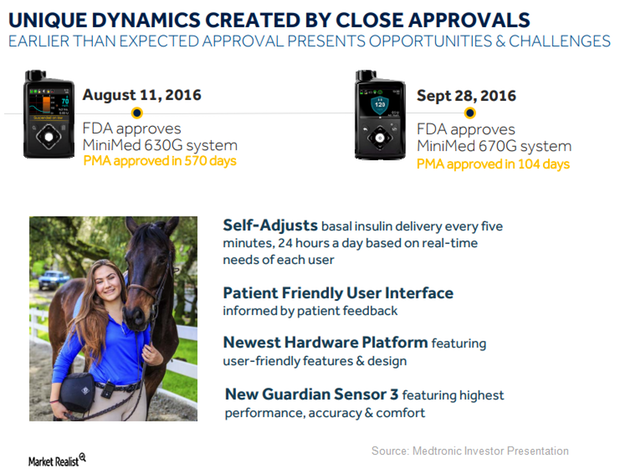
Medtronic Saw Several Challenges during Launch of MiniMed 670G
In fiscal 2H17, Medtronic launched the priority access program to first target those patients who were interested in purchasing MiniMed 670G.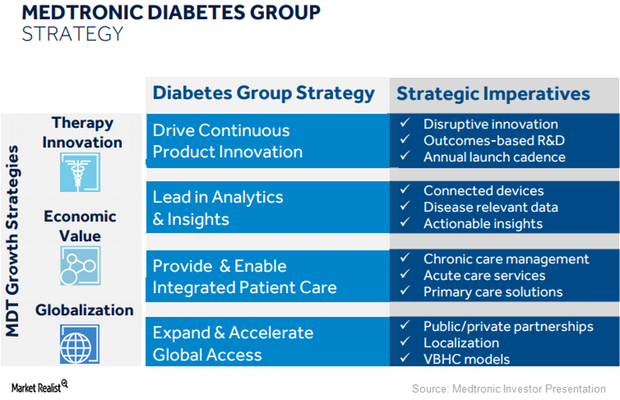
What’s Medtronic’s Long-Term Growth Strategy for Its Diabetes Business?
Medtronic (MDT) expects its diabetes business to witness a temporary sequential drop in revenues in 2Q18 and then return back to growth in the second half of fiscal 2018.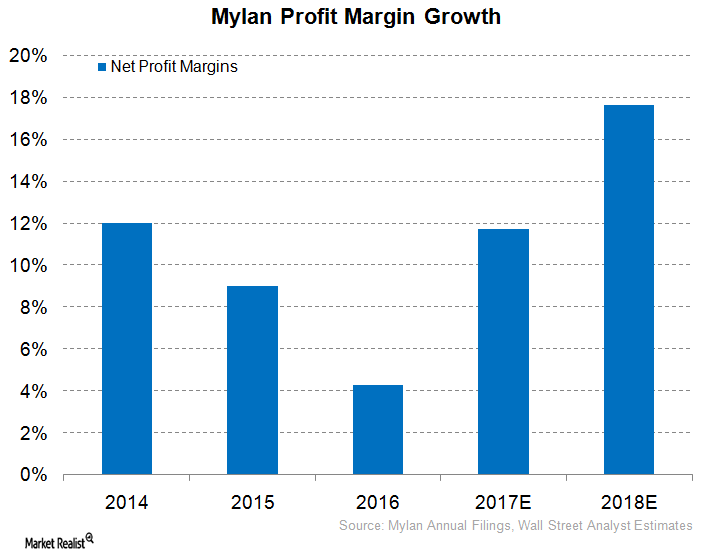
Could Mylan See a Rise in Net Profit Margins in 2017?
In 2Q17, Mylan (MYL) reported gross profit margins of 54.0%, which was lower than 56.0% reported in 2Q16.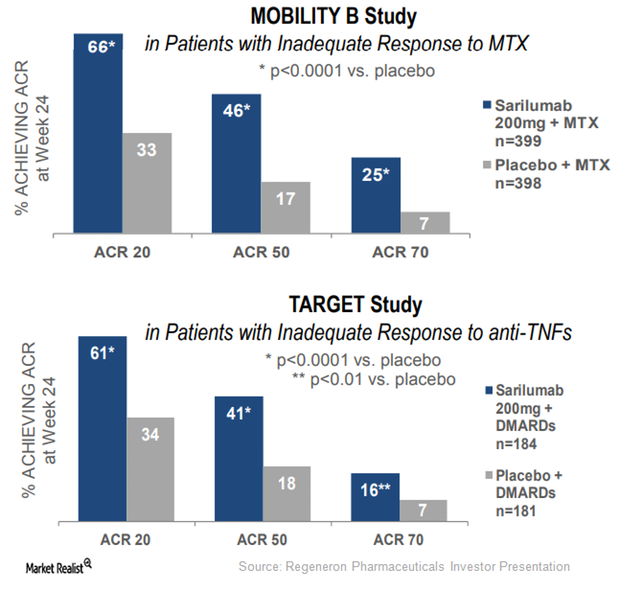
Kevzara May Emerge as a Prominent Rheumatoid Arthritis Drug in 2017
Regeneron and Sanofi have submitted an application seeking regulatory approval for Kevzara in Japan.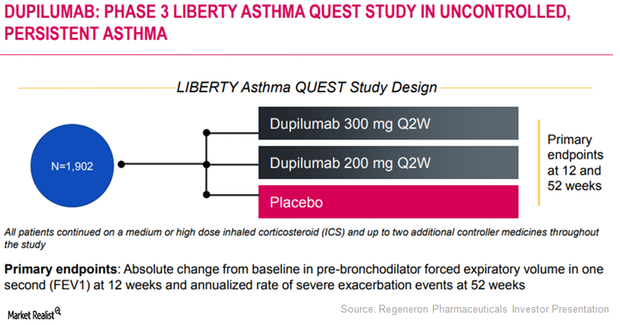
Regulatory Approval in Asthma Indication May Boost Dupixent’s Sales
In June 2017 and July 2017, healthcare providers wrote prescriptions for Regeneron (REGN) and Sanofi’s (SNY) Dupixent for 750 new patients on a weekly basis.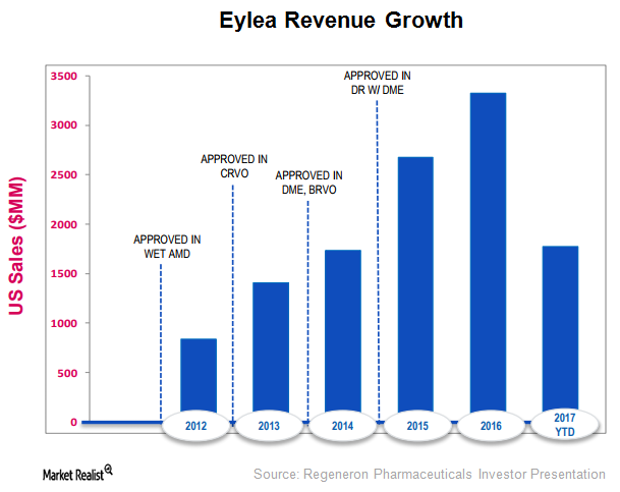
Eylea Leads the Retinal Diseases Sector
In 1H17, Eylea’s total sales rose 10% on a year-over-year basis.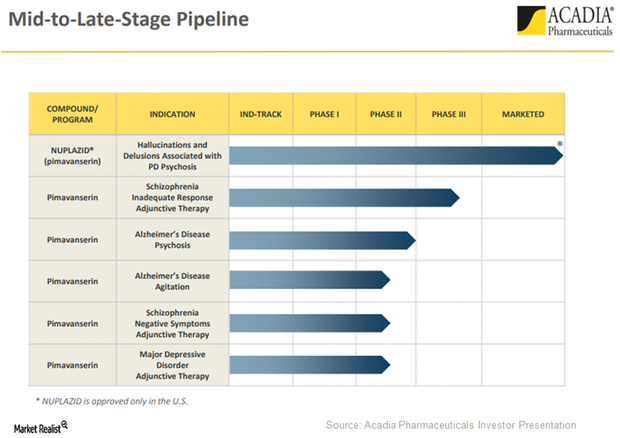
Nuplazid for Long-Term Care: Revenue Driver for Acadia?
Acadia Pharmaceuticals (ACAD) plans to focus on long-term care (or LTC) facilities to boost Nuplazid’s adoption in the Parkinson’s disease (or PD) psychosis indication.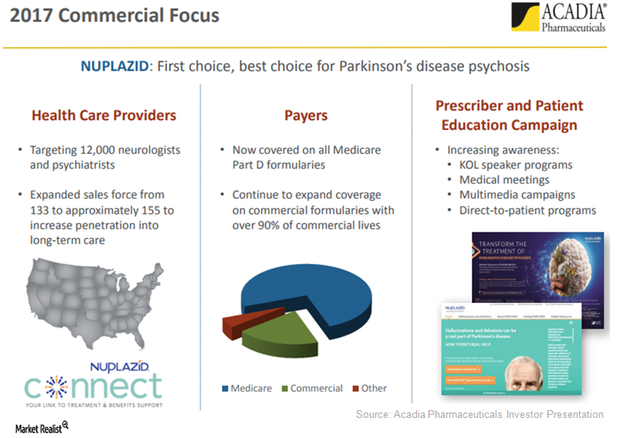
Nuplazid Sees Increasing Physician Intent to Prescribe in 2017
Acadia Pharmaceuticals’ (ACAD) commercial teams have been carrying out promotional efforts to create awareness for Nuplazid among physicians with the intent to have them prescribe the drug.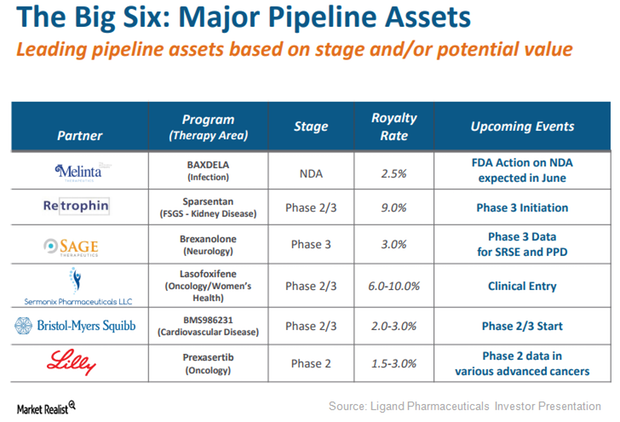
Captisol-Enabled Prexasertib May Be Major Growth Driver for Ligand
Eli Lilly’s (LLY) Captisol-enabled drug Prexasertib is currently being evaluated in multiple oncology indications such as head and neck cancer, small-cell lung cancer (or SCLC), and advanced metastatic cancer.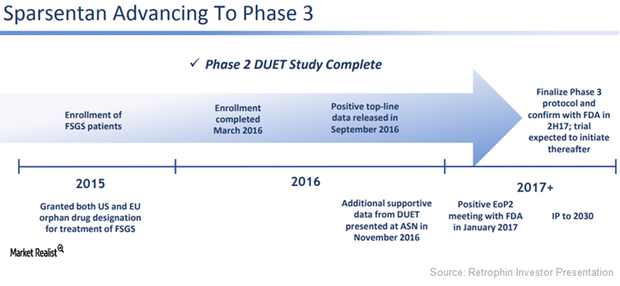
Sparsentan May Be Solid Near-Term Growth Driver for Ligand
With Sparsentan, Retrophin and Ligand Pharmaceuticals could become major nephrology players similar to peers such as Amgen (AMGN), AstraZeneca (AZN), and Bristol-Myers Squibb (BMY).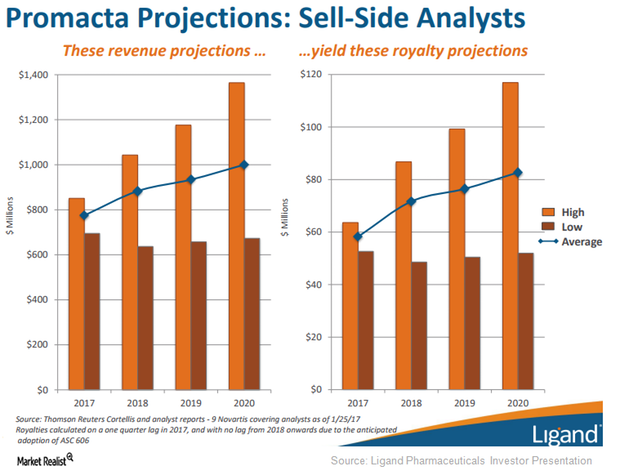
Ligand Pharmaceuticals Projects Higher Average Royalty Rate for Promacta
Ligand Pharmaceuticals (LGND) receives royalties from Novartis on Promacta’s sales according to a tiered annual payment structure.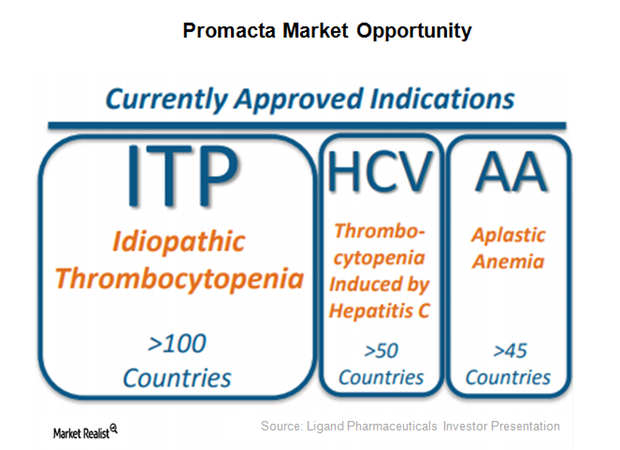
Promacta: A Major Growth Driver for Ligand Pharmaceuticals in 2017
Promacta was discovered by Ligand Pharmaceuticals (LGND) and GlaxoSmithKline (GSK) as a part of their thrombopoietin (or TPO) receptor agonist research collaboration.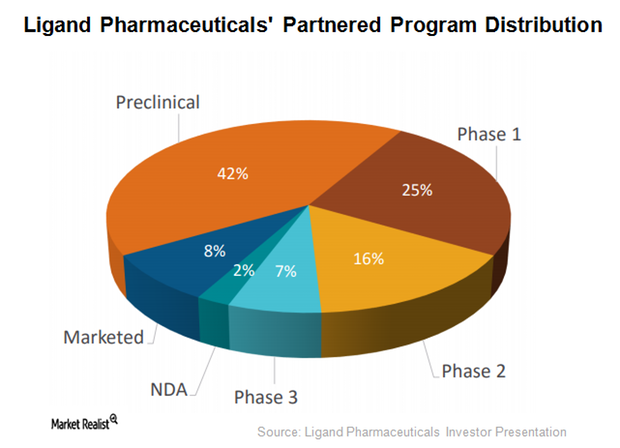
Fully Funded Partnerships May Drive Growth for Ligand Pharmaceuticals
Ligand Pharmaceuticals (LGND) expects its licensees to invest ~$2.0 billion for advancing more than 155 partnered research and development programs in 2017.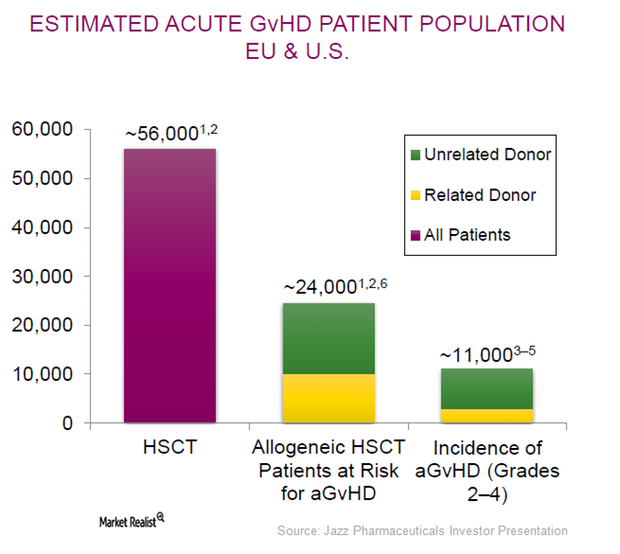
Jazz Pharmaceuticals Is Focused on Label Expansion of Defitelio
Defitelio could help Jazz Pharmaceuticals become a prominent player not only in the treatment but also in the prevention of post-HSCT complications.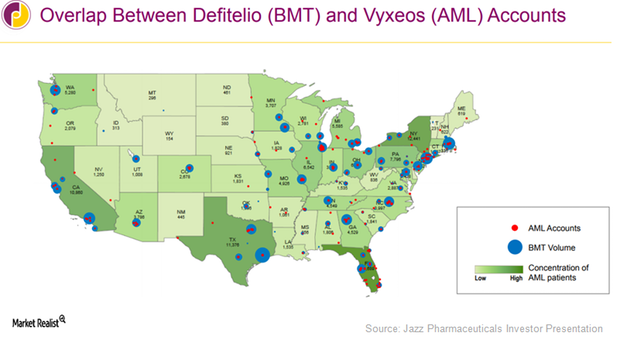
How Jazz Pharmaceuticals Aims to Boost Vyxeos Sales in 2017
In early 2017, Jazz Pharmaceuticals (JAZZ) expanded its sales force team from 35 to 55 and expanded its field reimbursement teams to support the commercial launch of Vyxeos in the US.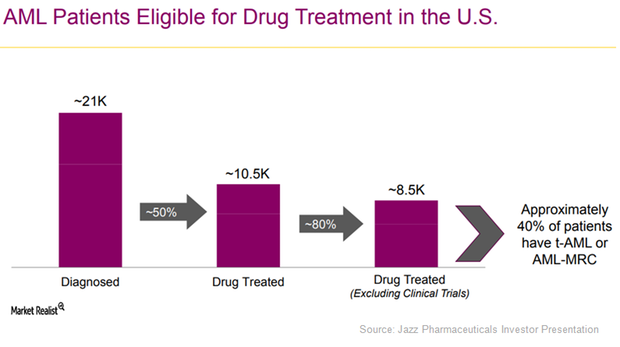
What Jazz Pharmaceuticals Expects for Vyxeos
If Vyxeos manages to penetrate a sizeable portion of the target market, it may have a favorable impact on Jazz Pharmaceuticals stock.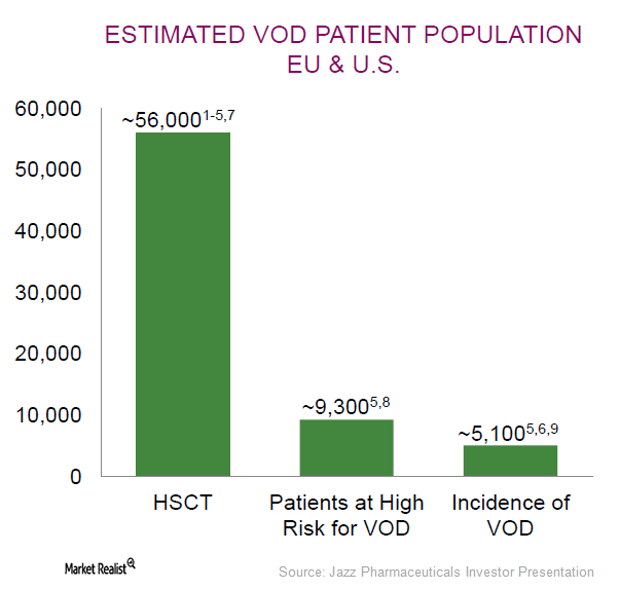
JAZZ Is Focused on Increasing Market Adoption of Defitelio
Jazz Pharmaceuticals (JAZZ) expects quarterly variability in Defitelio sales as the drug targets hepatic veno-occlusive disease (or VOD), an ultra-rare condition.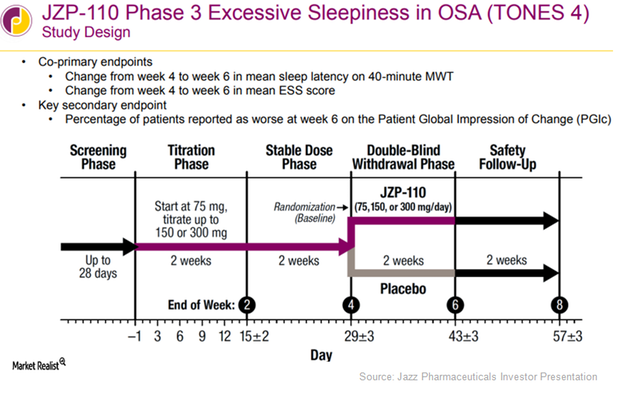
Inside the Efficacy of JAZZ’s JZP-110 in Obstructive Sleep Apnea Trials
This trial demonstrated the efficacy and safety of JAZZ’s JZP-110 in both the MWT and sleep latency tests at all dosages at the end of weeks 4 and 12.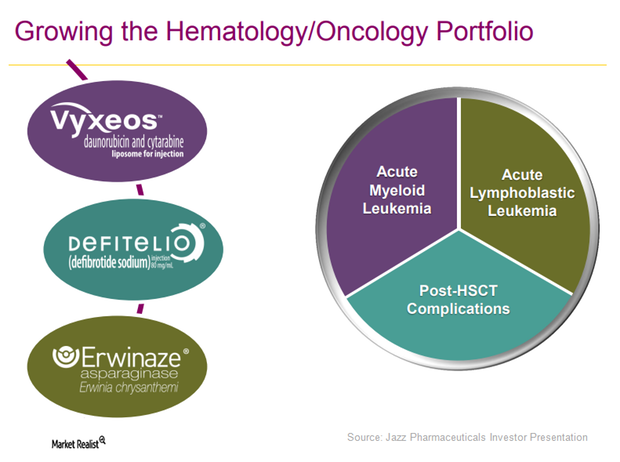
Erwinaze Sales May Remain Flat in 2017
Erwinaze saw net sales worth $49 million in 2Q17, almost flat compared to the drug’s revenues of $50 million in 2Q16.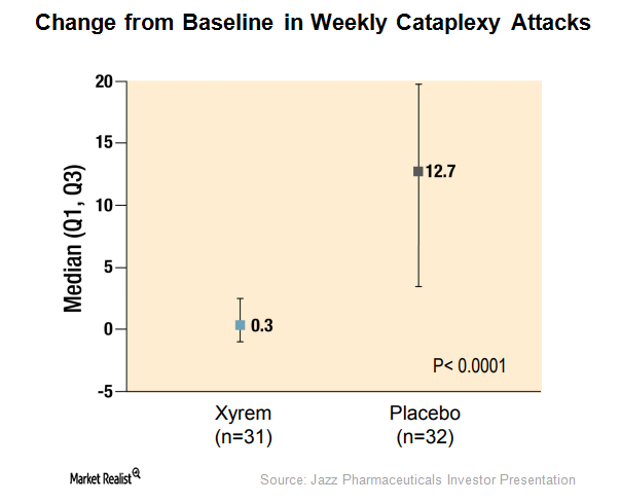
This Alone Could Boost Xyrem’s Addressable Market in 2018
To expand Xyrem’s addressable market, JAZZ has been evaluating the efficacy of Xyrem in pediatric narcolepsy patients with cataplexy and excessive sleepiness.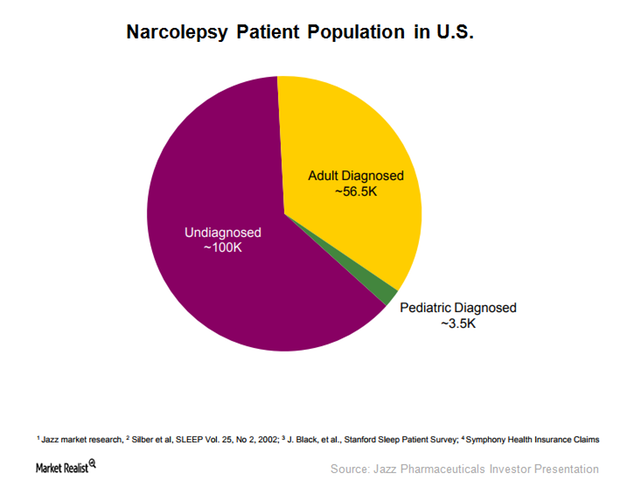
Behind Xyrem’s Solid Demand Trends in 2017
In 2Q17, Jazz Pharmaceuticals’ (JAZZ) Xyrem reported revenues of ~$298 million, which represented a YoY (year-over-year) growth of ~6% and sequential growth of ~9%.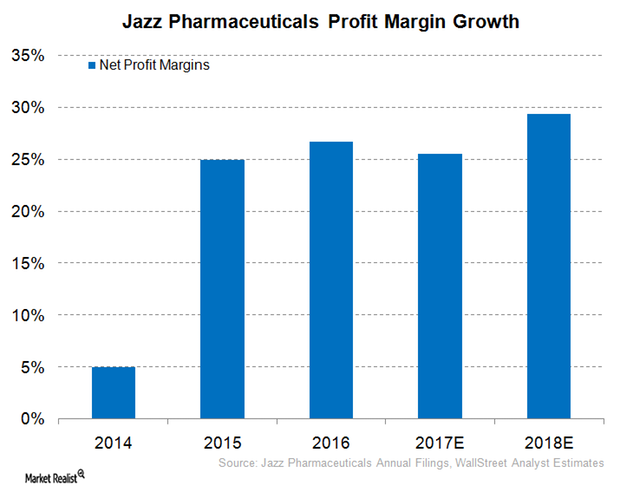
Inside Jazz’s Net Profit Margin Expectations for 2017
For 2Q17, Jazz Pharmaceuticals (JAZZ) reported revenues close to $394 million, which represented a YoY growth of ~3% and sequential growth ~5%.
North America: Fresenius Medical Care’s Major Target Market in 2017
In 2Q17, Fresenius Medical Care (FMS) reported revenues close to 3.3 billion euros, which represents year-over-year growth of ~11%.
Align Technology Focuses on International Market Strategy
International market strategy In 2Q17, Align Technology (ALGN) reported 85,400 Invisalign case shipments in international markets, which is YoY (year-over-year) growth of ~37.4% and sequential growth of ~13.6%. This growth was mainly attributed to new customers in the EMEA (Europe, the Middle East, and Africa) and Asia-Pacific markets. If Align Technology continues to demonstrate solid […]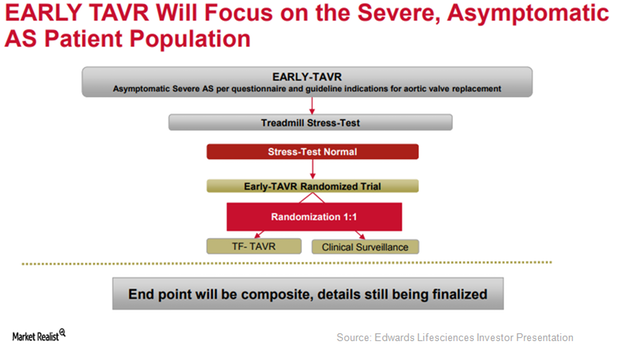
Edwards Lifesciences Focuses on Indication Expansion of SAPIEN 3 in 2017
To further expand the label of its transcatheter heart valve (or THV), SAPIEN 3, Edwards Lifesciences (EW) is currently involved in enrolling patients in its EARLY-TAVR trial.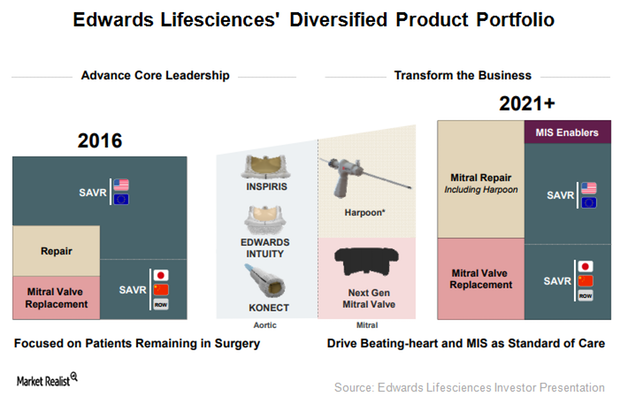
Edwards Lifesciences’ Mitral Regurgitation Segment
To diversify its focus beyond aortic structural heart conditions, Edwards Lifesciences (EW) acquired CardiAQ Valve Technologies in August 2015.
Edwards Lifesciences Focuses on Launch of SAPIEN 3 Ultra and CENTERA Valves
Edwards Lifesciences’ (EW) SAPIEN 3 Ultra system is a next-generation platform, with expandable Axela sheath technology and on-balloon delivery design.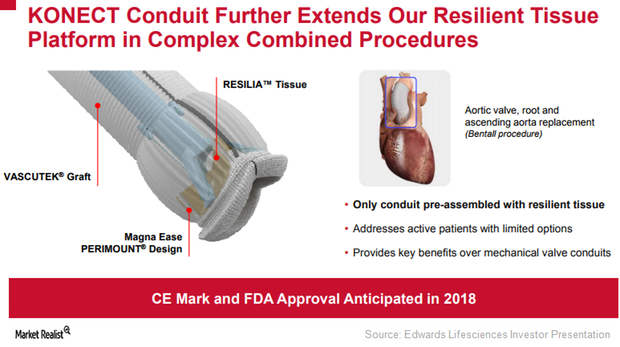
Edwards Lifesciences: Focused on Inspiris Resilia and Konect
To extend the durability of the Inspiris Resilia surgical aortic valve, Edwards Lifesciences (EW) has incorporated a new tissue platform, Resilia tissue.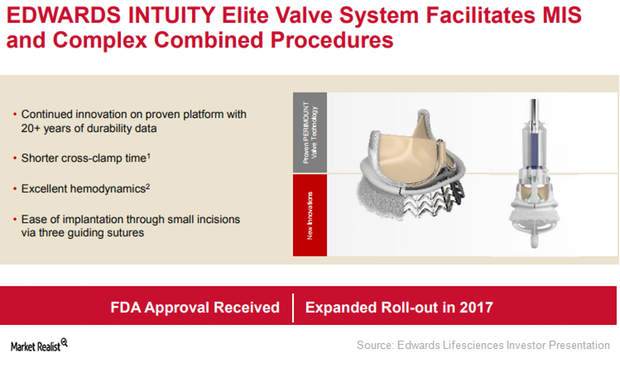
Can Edwards Intuity Elite Boost Edwards Lifesciences’ Revenues?
With the Edwards Intuity Elite valve system, Edwards Lifesciences aims to offer a minimally invasive therapy to complex aortic stenosis patients.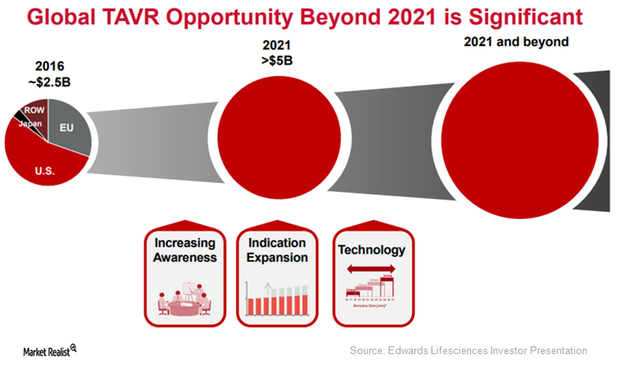
Transcatheter Heart Valve Therapy: Growth Driver for Edwards Lifesciences
In 2Q17, Edwards Lifesciences’ (EW) Transcatheter Heart Valve Therapy segment reported revenues close to $316 million, which represents year-over-year growth of ~28%.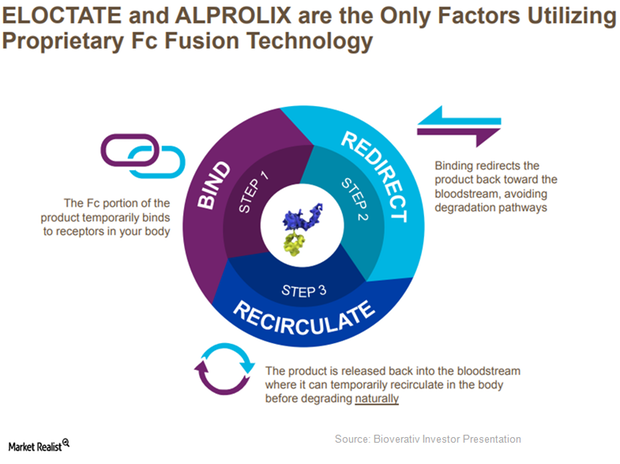
Alprolix and Eloctate Increasingly Used for Prophylaxis in 2017
Since demand from the target population for prophylaxis is rising rapidly, Bioverativ’s Alprolix and Eloctate are expected to see solid demand trends in 2017.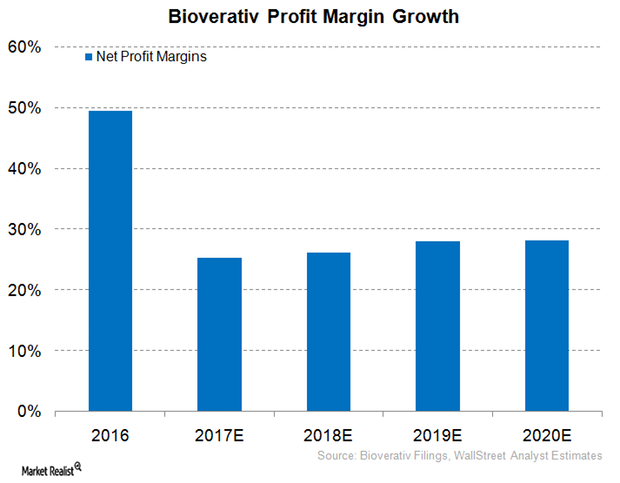
Bioverativ Expected to Report Healthy Profit Margins in 2017
Bioverativ (BIVV) expects its 2017 GAAP and non-GAAP operating margins to fall 38.0%–42.0% and 43.0%–47.0%, respectively.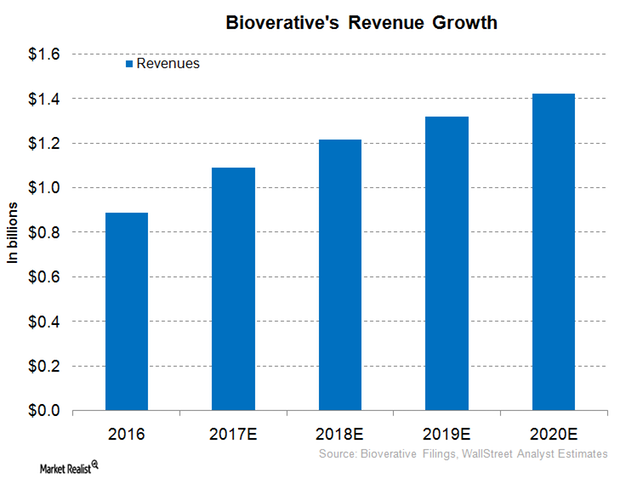
Bioverativ Expected to Report Robust Revenue Growth in 2017
In 1Q17, Bioverativ reported revenues close to $259.0 million, driven by its focus on a commercial strategy for its hemophilia products and optimal cost management.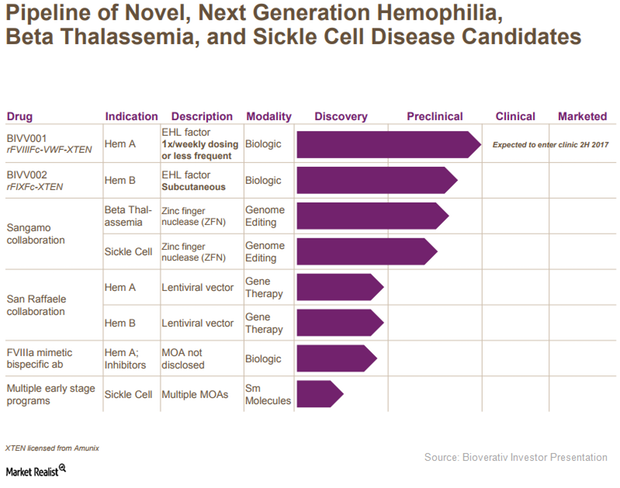
Bioverativ’s Research Pipeline May Boost Future Revenues
Bioverativ (BIVV) plans to improve compliance rates for hemophilia patients further by launching therapies with lower dosage frequency.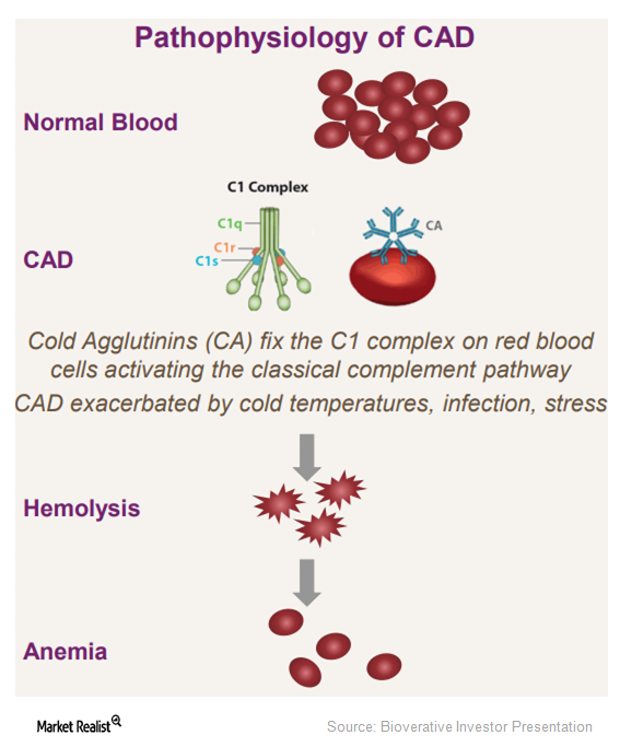
Bioverativ–True North Therapeutics: Stronger Research Pipeline
The acquisition of True North Therapeutics has paved the way for Bioverativ’s (BIVV) entry into cold agglutinin disease (or CAD).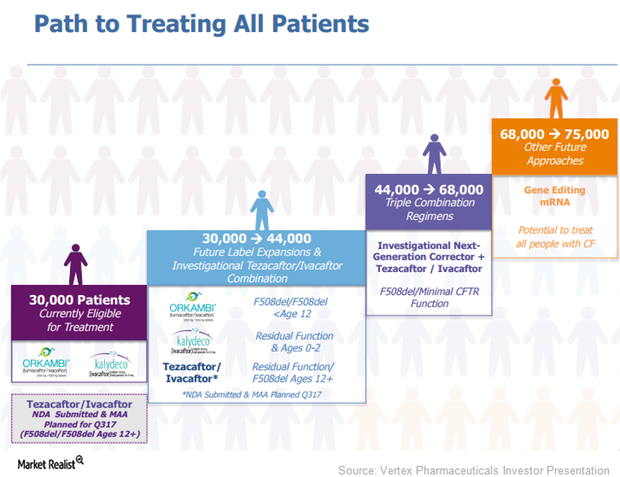
Vertex Pharmaceuticals Cystic Fibrosis Market Could Expand
Vertex Pharmaceuticals’ (VRTX) drugs, Orkambi (lumacaftor/ivacaftor) and Kalydeco (ivacaftor), are capable of treating around 30,000 cystic fibrosis (or CF) patients.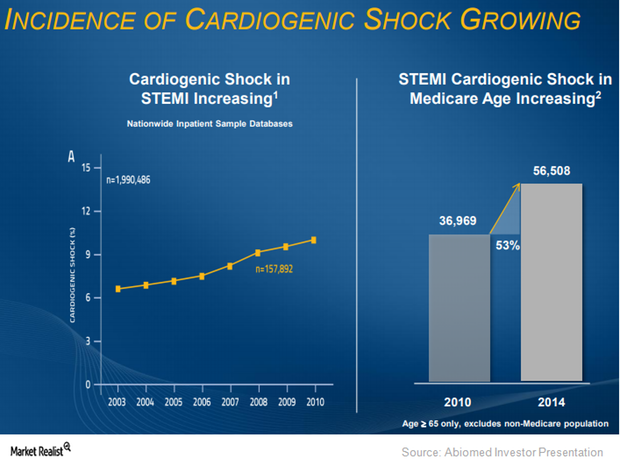
Where Abiomed Plans to Expand Impella CP’s Label
About 40% of patients succumb to heart failure within five years. Abiomed believes that it is reperfusion injuries that cause these problems.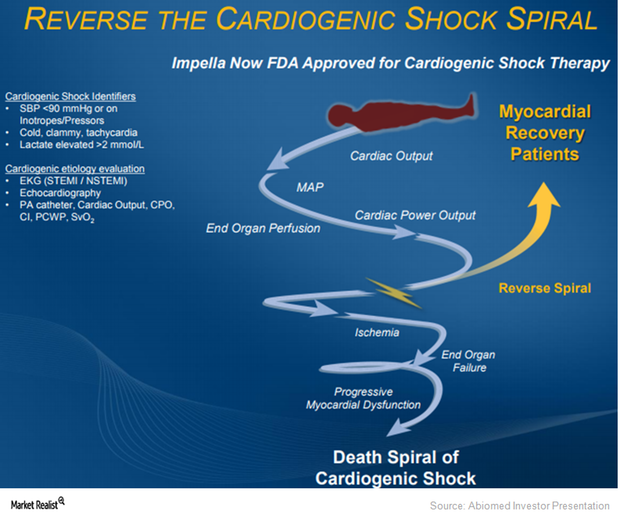
Why Abiomed Expects to Benefit from Impella in Cardiogenic Shock
On March 23, 2015, the FDA approved Abiomed’s Impella 2.5 heart pump as a temporary ventricular support device.
