
Margaret Patrick
Margaret Patrick joined Market Realist in September 2014 and has written close to 3,000 articles. She has covered the healthcare sector, which includes pharmaceutical and biotechnology companies, medical device companies, health insurance companies, and hospital companies. Currently, she is following the cannabis sector.
Prior to joining Market Realist, Margaret worked as equity and data analyst at MSCI for a year and as a financial research analyst at Deloitte for two years. She completed her MBA with finance specialization in 2011. She also passed all three CFA levels.
Besides writing on stocks, Margaret loves to read about nutrition, culture, and mythology. She's also fond of traveling to new places.
Disclosure: I am in full compliance with all ethics and other policies for Market Realist research analysts. I am not invested in securities that I cover on Market Realist.
More From Margaret Patrick
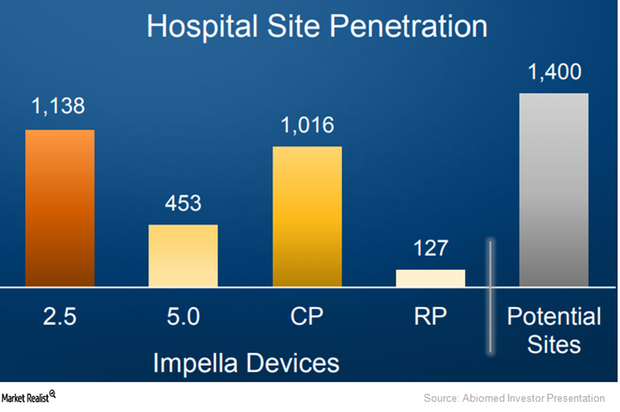
Behind Abiomed’s Plans to Create a Greater Awareness of Impella
To target patients across communities, Abiomed (ABMD) has been developing a hub-and-spoke model with hospitals.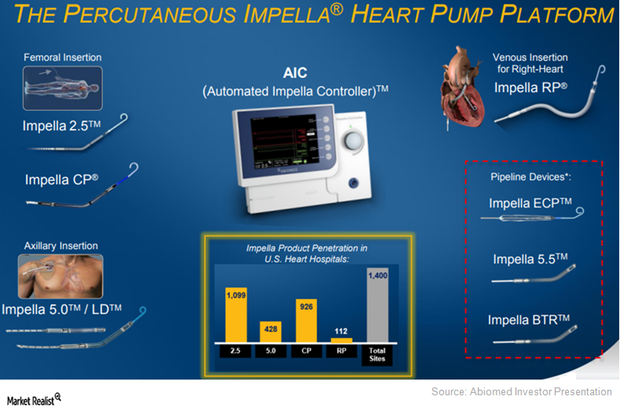
Abiomed Aims to Expand Impella RP’s Penetration Going Forward
In March 2017, Abiomed (ABMD) submitted premarket approval (or PMA) to the FDA for its Impella RP device far ahead of schedule.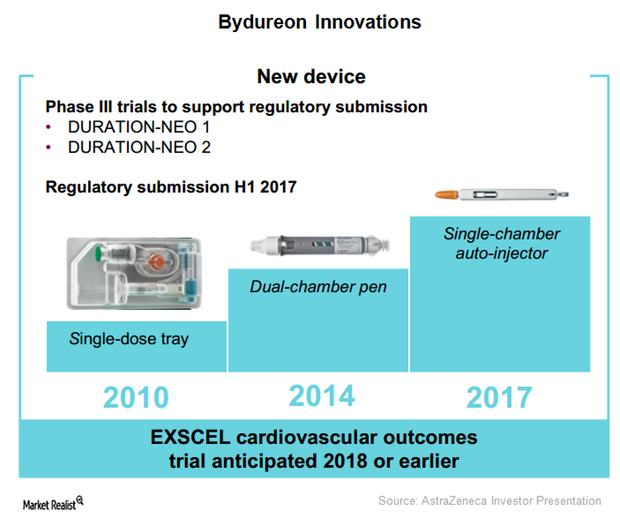
Bydureon Auto-Injector May Boost the Drug’s Sales in Future Years
The combined product sales of Bydureon and Byetta globally in 1Q17 was ~$199 million, which represents 1% YoY growth on a reported basis.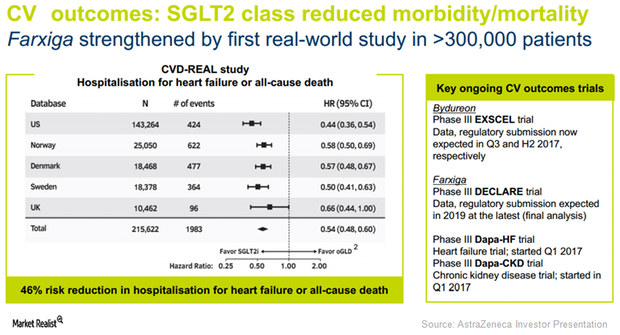
Farxiga Could See Robust Demand in International Markets in 2017
In 1Q17, AstraZeneca’s (AZN) Farxiga reported sales of $42 million in emerging markets, which equals year-over-year growth of ~100% on a reported basis.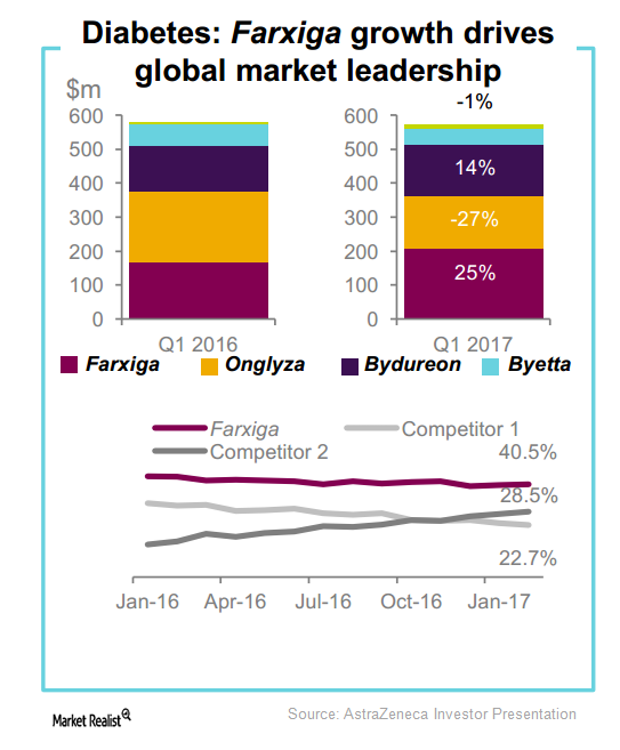
Farxiga Expected to Drive AstraZeneca’s Performance in Diabetes Segment
In 1Q17, AstraZeneca’s leading sodium-glucose co-transporter 2 (or SGLT2) therapy for type-2 diabetes, Farxiga, reported revenues close to $270 million. This represents YoY growth of ~25% on a constant currency basis.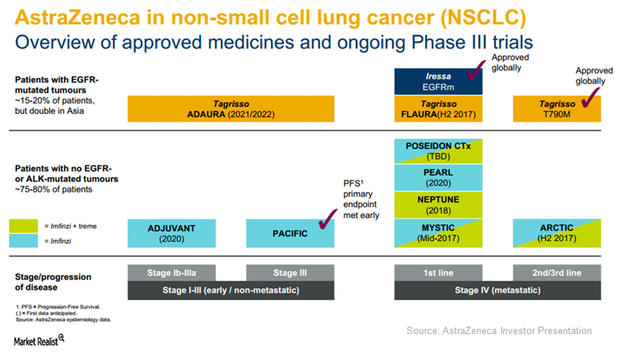
AstraZeneca Aims to Offer Multiple Therapies in NSCLC Segment
EGFR mutant NSCLC has become a major market opportunity for AstraZeneca’s drugs Tagrisso and Iressa.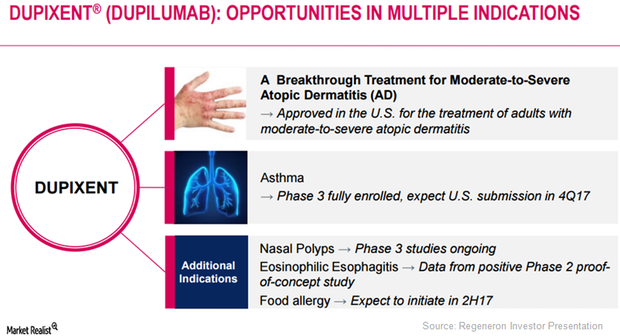
Eosinophilic Esophagitis: Major Market Opportunity for Dupixent?
Regeneron (REGN) has obtained positive results from its Phase 2 proof-of-concept study evaluating Dupixent in eosinophilic esophagitis.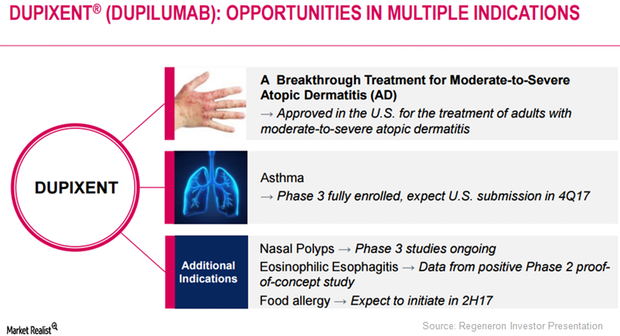
Dupixent May Prove Effective in Multiple Diseases
Regeneron plans to discuss Dupixent’s innovative mechanism-based treatment approach with regulatory authorities.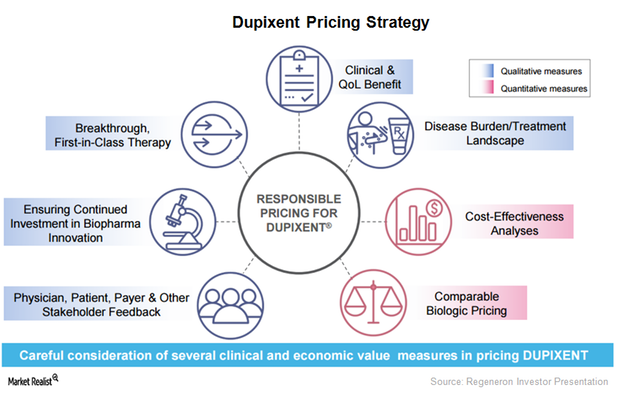
Dupixent May Be a Major Growth Driver for Regeneron in 2017
After Dupixent’s commercial launch, Regeneron has been involved in creating awareness for the drug among physicians who have been treating AD patients.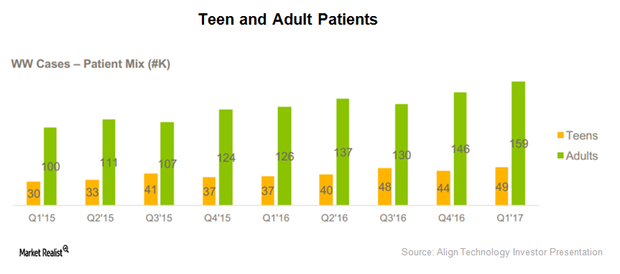
Inside Align Technology’s Market Expansion Strategy
In 1Q17, Align Technology (ALGN) also witnessed a robust rise of around 45.2% in volumes of Invisalign sold in Asia-Pacific markets on a YoY basis.
These Are Align Technologies Key Demand Drivers in North America
In 1Q17, Align Technology (ALGN) has started selling its new product Invisalign Go in North America, which involves a few DSOs (dental service organizations).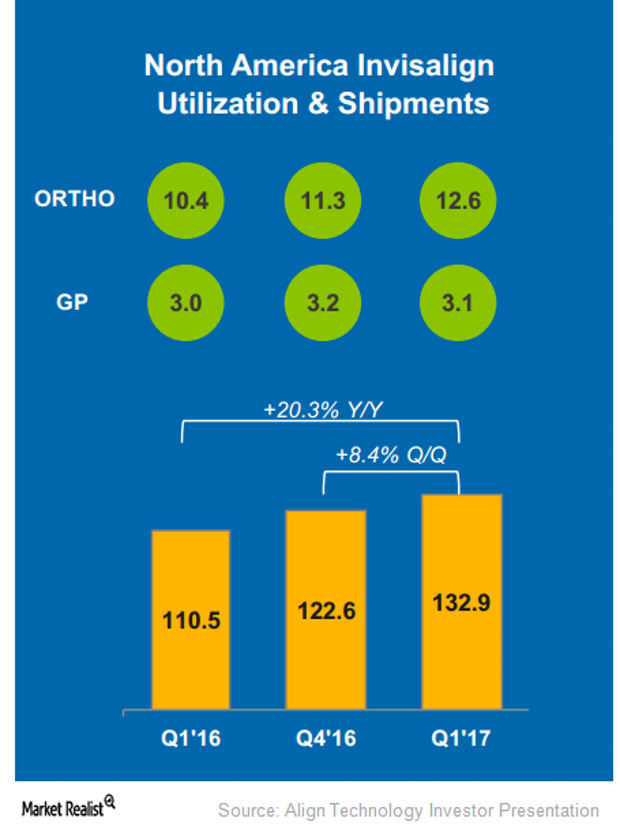
Behind Align Technology’s North American Expectations for 2017
In 1Q17, Align Technology’s (ALGN) Invisalign sales volumes in North American markets rose YoY by 20.3% and QoQ by 8.3%.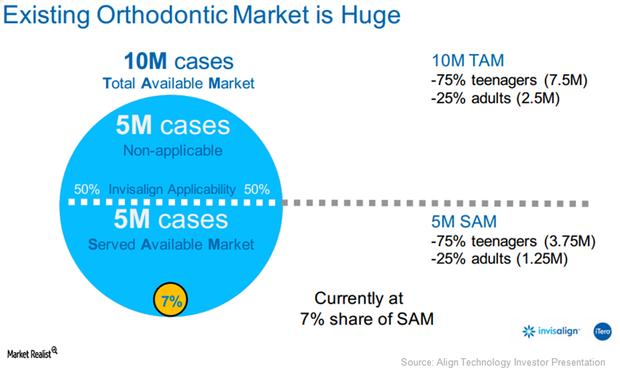
Why Align Technology’s Orthodontics Is a Solid Growth Opportunity in 2017
Align Technology (ALGN) is a medical device provider focused on malocclusion or teeth misalignment condition.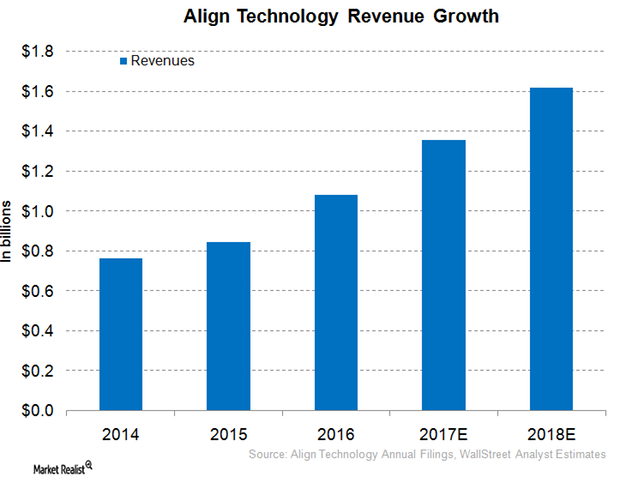
Inside Align Technology’s Robust Revenue Growth Projection for 2017
For fiscal 2017, Align Technology (ALGN) expects its 2017 revenues to grow operationally in the range of 15%–25% YoY.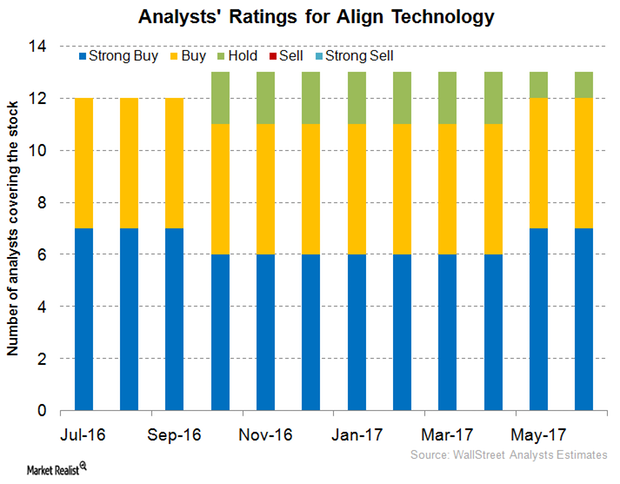
Align Technology versus Peers in June 2017: Analyst Recommendations
For 1Q17, Align Technology (ALGN) reported revenues close to $310.3 million, which represents YoY (year-over-year) growth of around 30.0%.
What Bio-Rad Laboratories Expects from Life Science
In 1Q17, Bio-Rad Laboratories’ (BIO) Life Science segment reported revenues of ~$174.3 million, which represents a YoY rise of ~5.1%.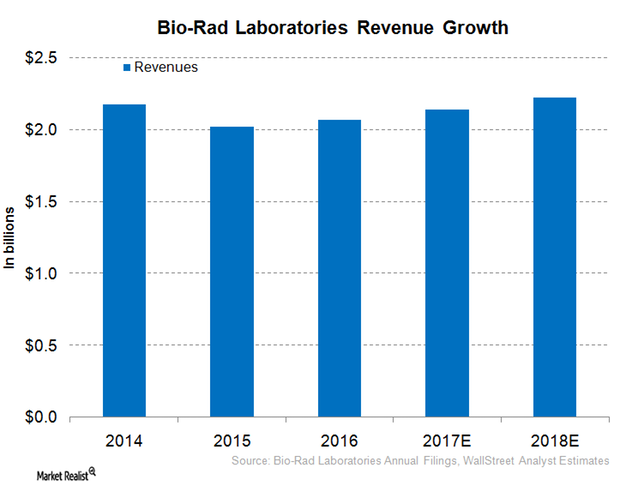
Inside Bio-Rad Laboratories’ Robust Revenue Growth Projection for 2017
On March 13, 2017, Bio-Rad Laboratories (BIO) provided a long-term, currency-neutral revenue growth target of around 3%–5%.
Why Zoetis’s Apoquel and Cytopoint Are Expected to Report Robust Revenues
In 1Q17, Zoetis’s (ZTS) dermatology portfolio reported revenues of ~$57 million, driven by the steady adoption of Cytopoint and Apoquel.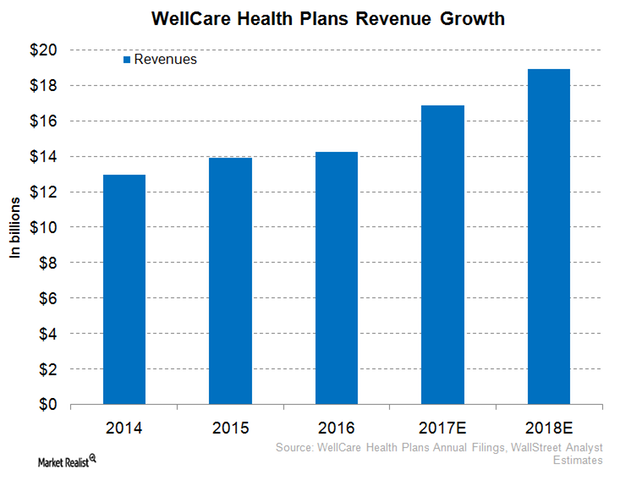
WellCare Health Plans Expects Robust Revenue Performance in 2017
In 1Q17, WellCare Health Plans (WCG) reported revenues of ~$3.9 billion, which totals year-over-year growth of around 11.7%.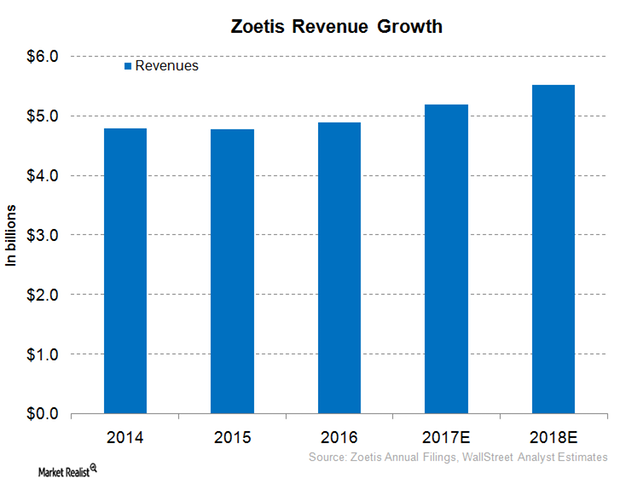
Why Zoetis Expects to See Robust Revenue Growth in 2017
Zoetis (ZTS) has adopted a targeted R&D strategy that has resulted in multiple new product launches.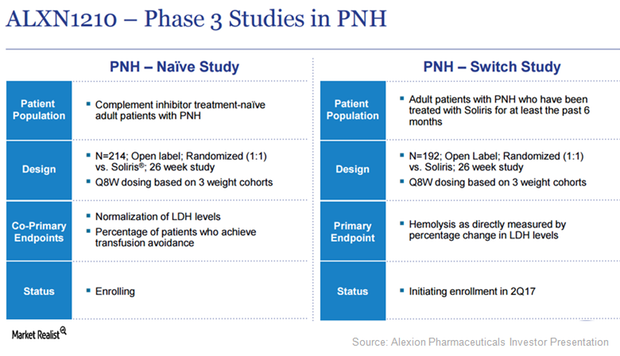
ALXN1210 Is Expected to Boost Alexion Pharmaceuticals’ Revenue
Alexion Pharmaceuticals has planned five clinical trials in 2017 to test the potential of investigational next-generation C5 antibody, ALXN1210, as a treatment option for complement-mediated diseases.
Biogen’s Targeted Marketing Strategy for Spinraza in 1Q17
To promote the use of Spinraza for SMA, Biogen (BIIB) has been actively educating and creating awareness for the drug among physician and patient communities.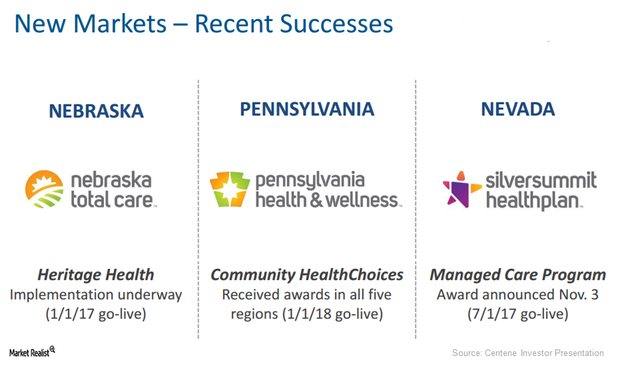
Centene Saw Robust Rise in Medicaid Enrollments in 1Q17
In 1Q17, around 12.1 million members were enrolled in Centene’s (CNC) various healthcare plans, which is a year-over-year (or YoY) rise of around 600,000 beneficiaries.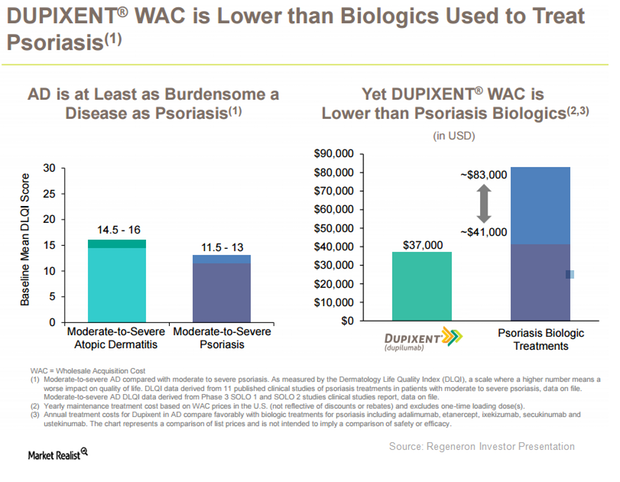
Dupixent Expected to Be a Solid Addition to Regeneron’s Portfolio
On March 28, 2017, the FDA approved Regeneron (REGN) and Sanofi’s (SNY) Dupixent for the treatment of patients with moderate-to-severe eczema or atopic dermatitis (or AD).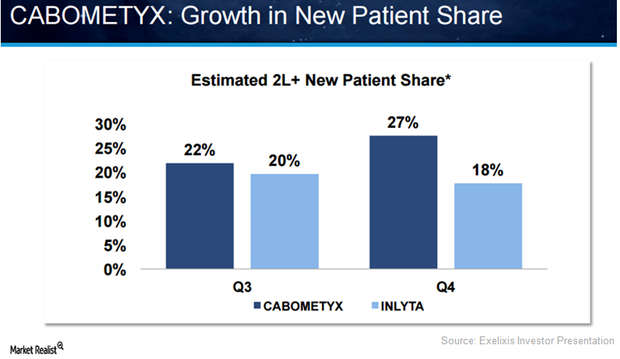
Behind Exelixis’s Successful Commercial Launch of Cabometyx in 2016
Launched in the US in 2Q16, Exelixis’s (EXEL) Cabometyx managed to fetch revenues of $31.2 million and $44.7 million in 3Q16 and 4Q16, respectively.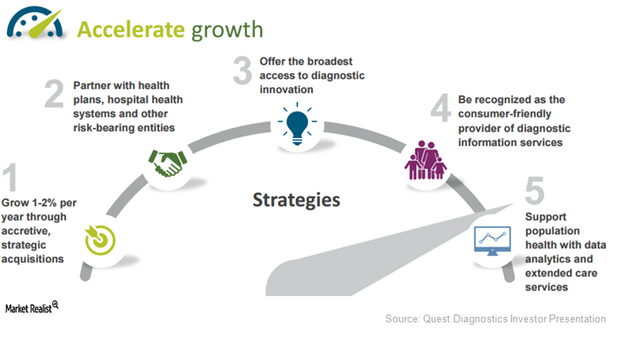
Quest Diagnostics’ Multi-Pronged Strategy to Accelerate Growth
Quest Diagnostics (DGX) expects to witness a 3.0%–5.0% long-term revenue growth rate in the future with earnings growth of 5.0%–9.0%.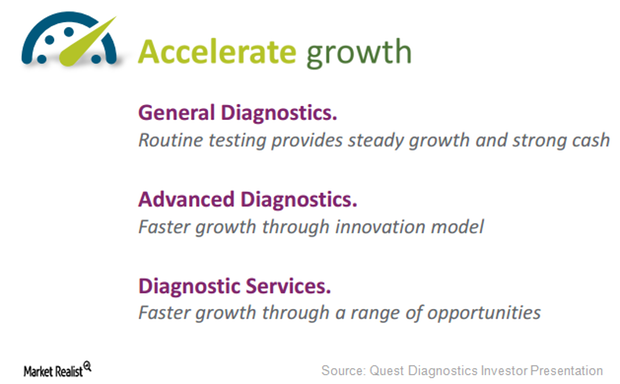
Quest Diagnostics Is Targeting 3 Focus Areas to Accelerate Growth
Since 2012, Quest Diagnostics (DGX) has spent about $1.0 billion on capital investments and $1.0 billion on ten acquisitions to support inorganic growth.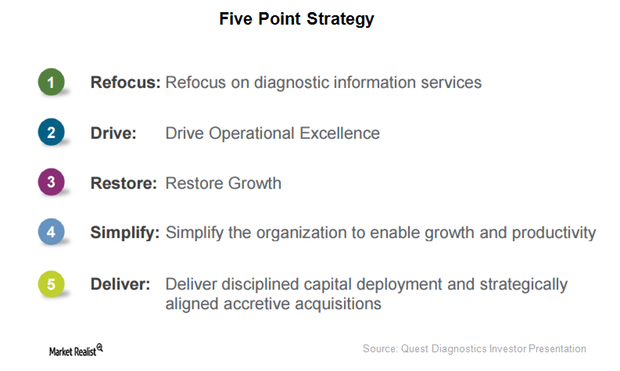
Quest Diagnostics Is Making Steady Progress in 5-Point Strategy
Since 2012, Quest Diagnostics (DGX) has been working on a five-point strategy to boost revenue growth and improve quality, service, and efficiency.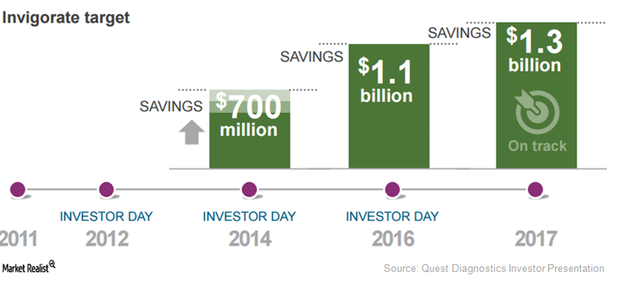
Quest Diagnostics Projects Run Rate Savings by End of 2017
Quest Diagnostics (DGX) has projected a run rate savings worth $1.3 billion by the end of 2017. In 2016, it managed to save up to $1.1 billion.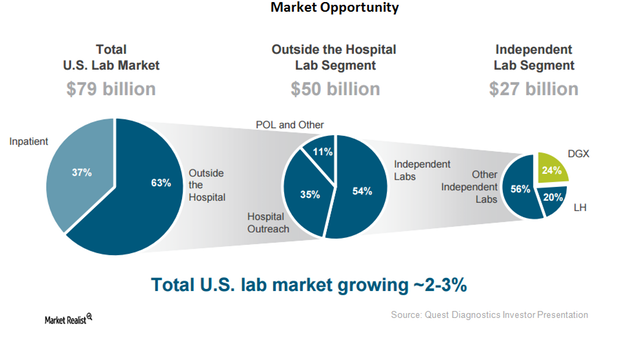
Quest Diagnostics Could Get Greater Share of Laboratory Segment
With around 50.0% of hospitals being served by Quest Diagnostics (DGX), the company has become a leading player in the fragmented US laboratory market.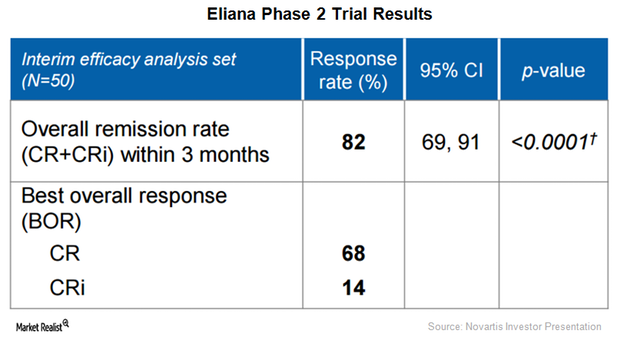
Could Novartis’s CTL019 Capture Significant Market Share?
Currently, an estimated 7,000 patients suffer from pediatric ALL in the US, Europe, Japan, Canada, and Israel.
Novartis Expects to Restore Alcon’s Profitability in the Future
Novartis (NVS) has focused its efforts on improving its customer service levels and entering into lucrative partnering deals to boost profitability for its Alcon business.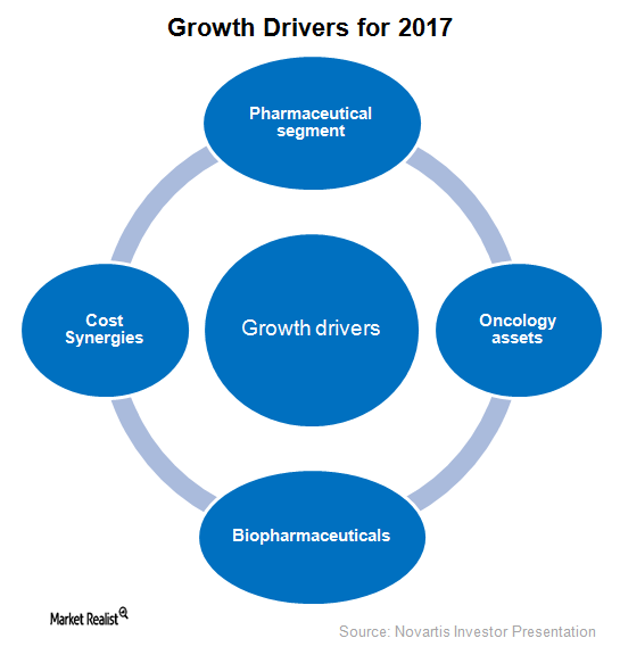
Novartis Expects to Witness Flat Revenue Growth in 2017
Novartis (NVS) has projected its 2017 revenue to be close to what it earned in 2016. The company also expects its core operating income to be flat or to fall in the single digits.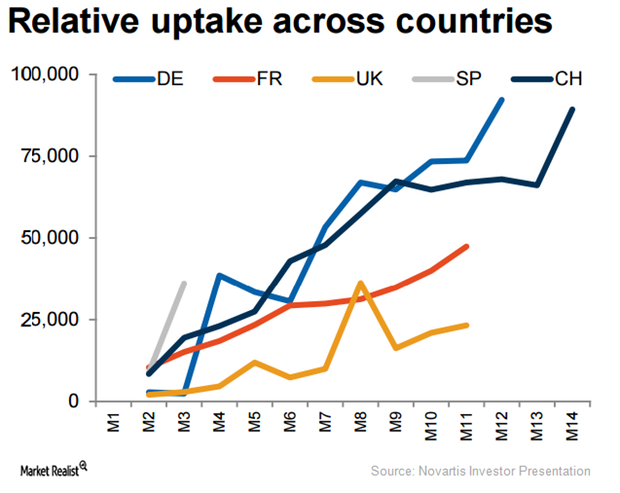
Entresto Could See Strong Uptake in European Markets in 2017
Novartis (NVS) has managed to secure reimbursement in 17 countries in Europe.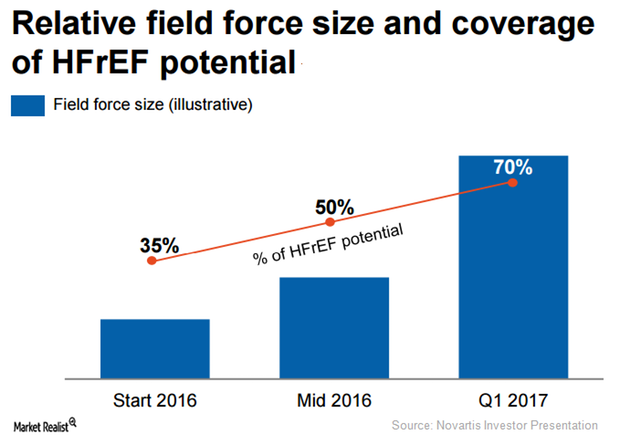
How Novartis Is Aiming to Increase Entresto Sales in 2017
In 2017, Novartis (NVS) expects its heart failure drug, Entresto, to earn revenues in excess of $500 million.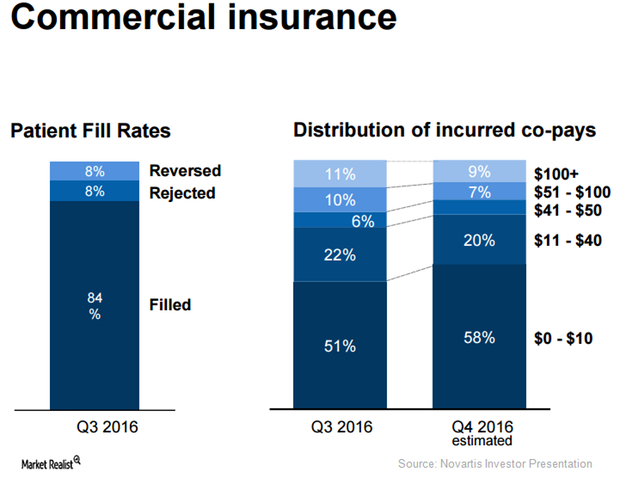
Reduced Co-pays May Boost Demand for Entresto
Compared to 43% at the end of 2015, Novartis’s (NVS) Entresto managed to attain a preferred formulary position in 66% of commercial plans in the US at the end of 2016.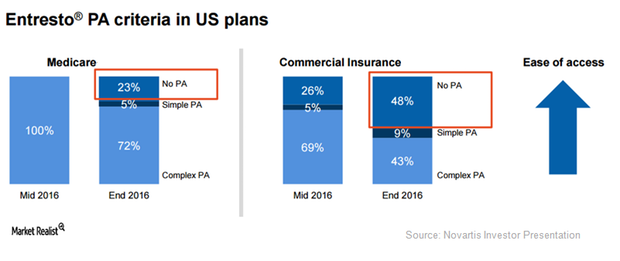
Why Entresto Could Become Key Growth Driver for Novartis in 2017
Novartis (NVS) expects modest prescription growth for its heart failure drug, Entresto, in 1Q17.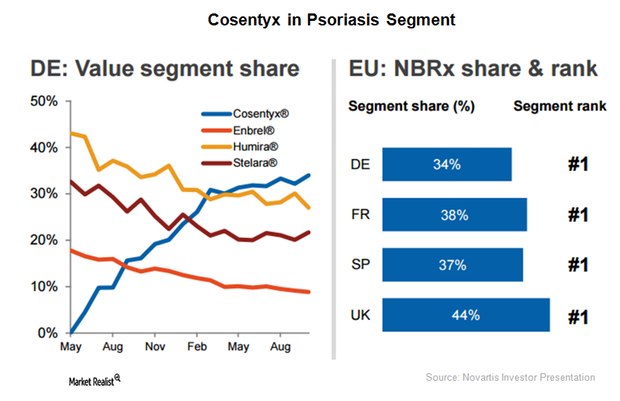
Cosentyx Enjoys Solid Demand as Psoriasis Therapy in Europe
In 2016, Novartis’s (NVS) Cosentyx surpassed its closest competitor, Eli Lilly’s (LLY) Taltz, in number of total weekly prescriptions in the US market.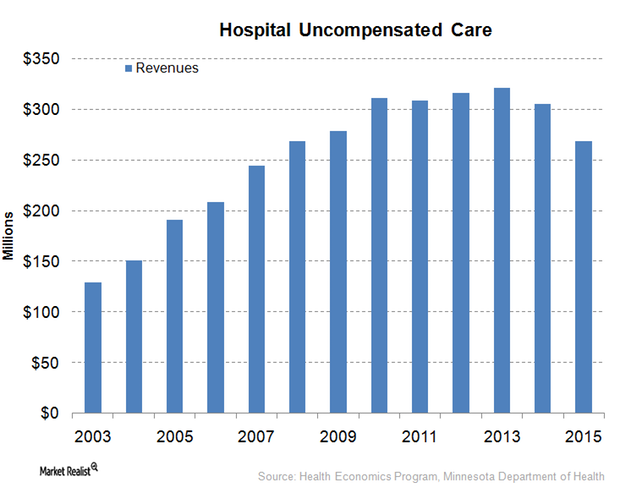
Hospital Industry Reacts to the Failed Healthcare Bill
On March 24, 2017, House Speaker Paul Ryan pulled back the American Health Care Act, also known as “Trumpcare,” before votes were cast.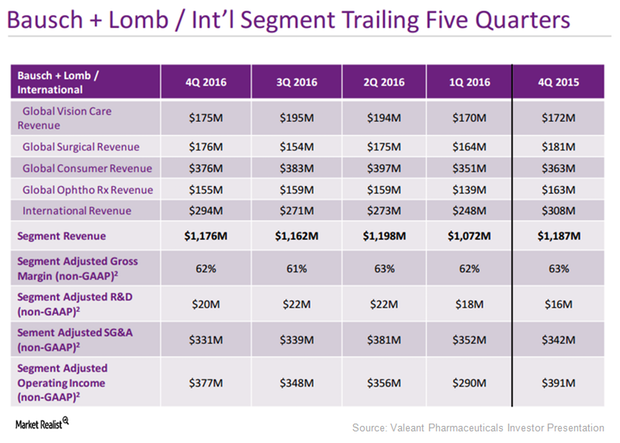
Bausch & Lomb Continues to be Key Growth Driver for Valeant
Bausch & Lomb projections Valeant Pharmaceuticals expects its Bausch & Lomb/International segment to grow at a CAGR (compound average growth rate) of 4%–6% between 2017 and 2020. In 2017, the company expects the segment to grow 5%–7% after adjusting for foreign exchange fluctuations and the impact of impending patent expiries. In 2017, the segment is also expected […]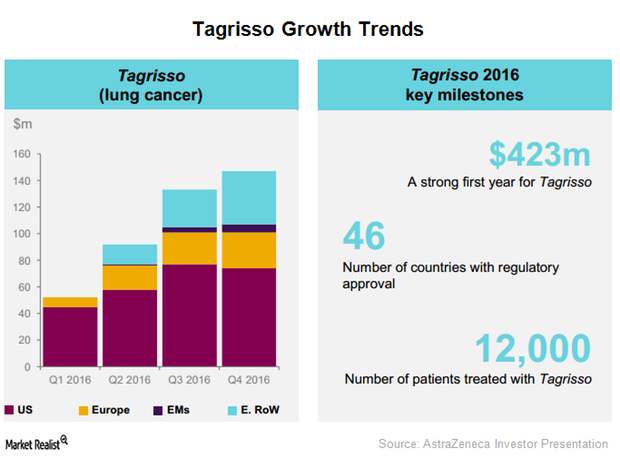
What Will Drive AstraZeneca’s Oncology Revenues in 2017?
In 2016, AstraZeneca’s (AZN) poly ADP-ribose polymerase (or PARP) inhibitor, Lynparza, managed to report revenues close to $218 million.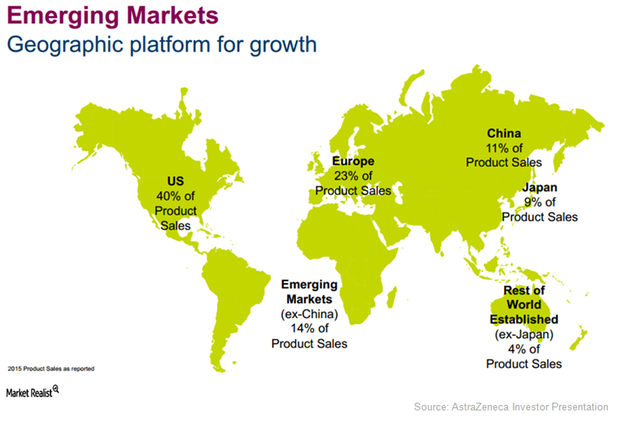
Emerging Markets Expected to Be AstraZeneca’s Key Growth Driver
For 2016, AstraZeneca (AZN) reported revenues of about $5.8 billion for its emerging markets business, which is a YoY (year-over-year) rise of about 6.0%.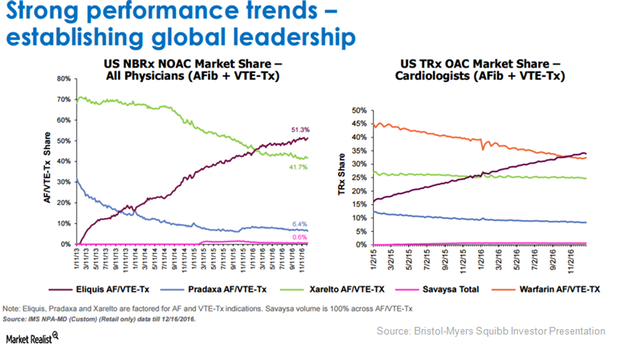
Bristol-Myers Squibb’s Profitability: Can Eliquis Do the Job in 2017?
BMY’s Eliquis can be considered a leading therapy for US patients suffering from Afib (atrial fibrillation) and venous thromboembolism.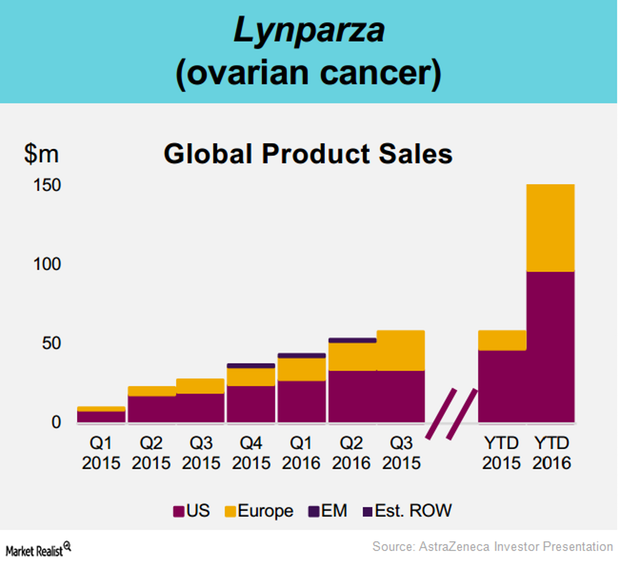
AstraZeneca’s Lynparza Is Still a Leading PARP Inhibitor in 2016
On October 26, 2016, AstraZeneca (AZN) announced that Lynparza managed to demonstrate a superior clinical profile in the Phase 3 SOLO-2 trial.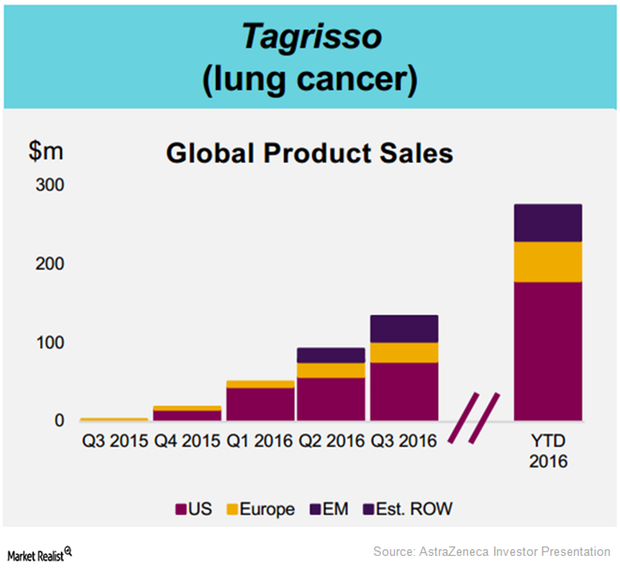
AstraZeneca’s Tagrisso Had a Strong Commercial Launch in 2016
After its commercial launch in 41 countries, AstraZeneca’s Tagrisso has witnessed a solid uptake, earning revenues of $276.0 million in 2016 YTD.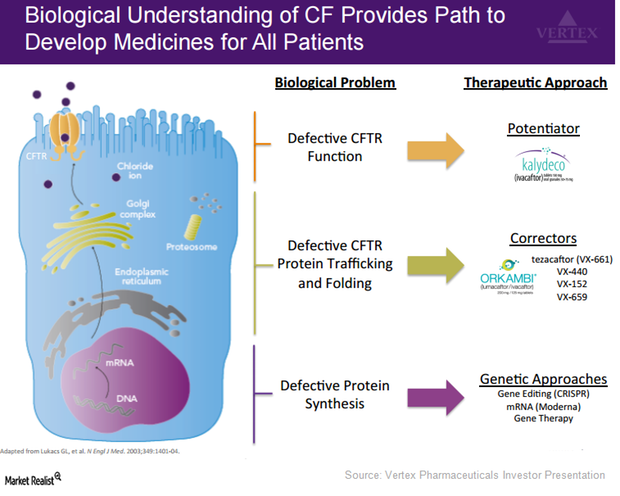
Vertex Has Adopted Multiple Approaches to Treat Cystic Fibrosis
Vertex Pharmaceuticals (VRTX) is aiming to increase the number of patients eligible to be treated with its drugs to include the entire CF patient population.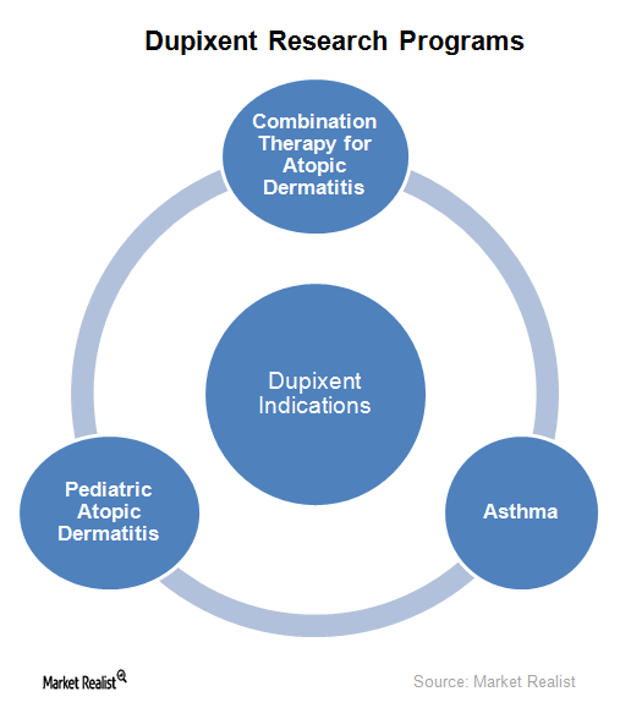
Dupixent Is Being Explored for Multiple Indications in 2016
In addition to being explored as a monotherapy for AD (atopic dermatitis), Regeneron and Sanofi are also researching Dupixent for other indications.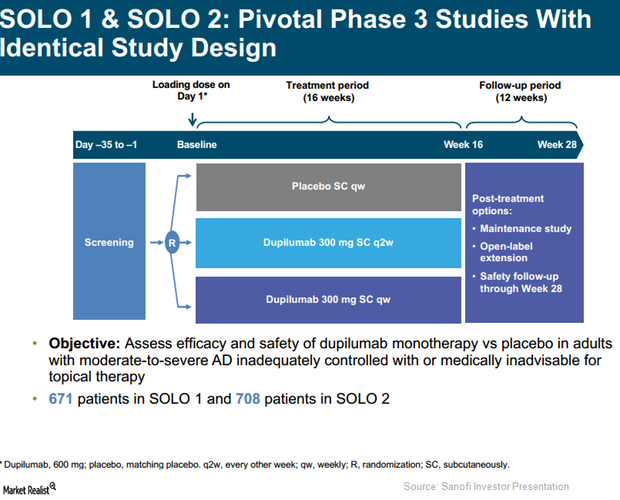
SOLO 1 and SOLO 2 Trials Could Strengthen Dupixent’s Label
In Phase 3 trials, SOLO 1 and SOLO 2, Regeneron (REGN) and Sanofi (SNY) tested the efficacy of an investigational therapy, Dupixent, compared to a placebo.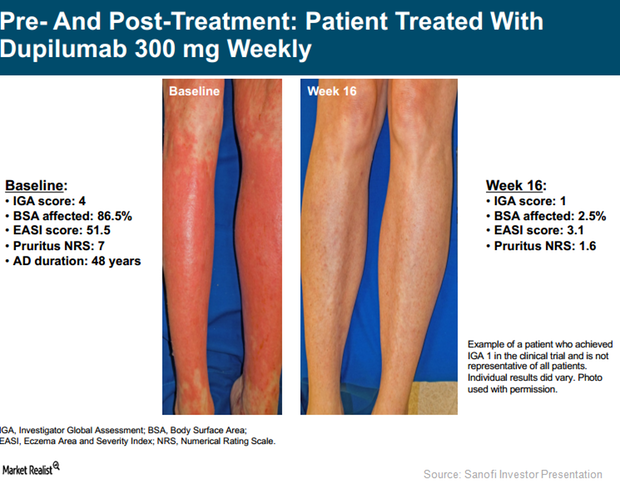
Dupixent: Leading Therapy for Atopic Dermatitis in the Future?
Existing treatment options for AD aren’t tolerated well by the entire patient population. Dupixent might become a preferred regimen in the future.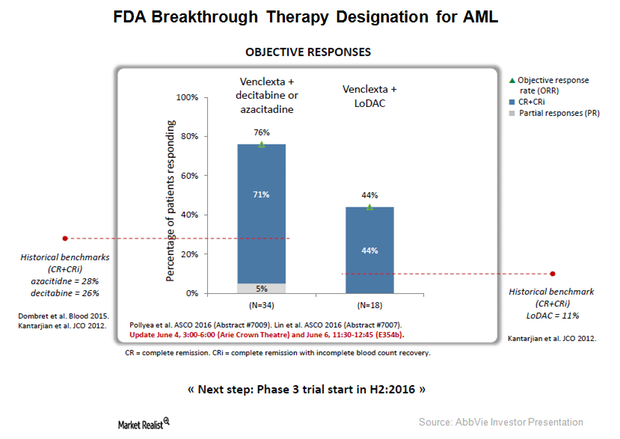
Acute Myeloid Leukemia: Growth Opportuny for AbbVie’s Venclexta?
AbbVie’s (ABBV) Venclexta has been granted FDA breakthrough therapy designation as a first line therapy for patients with acute myeloid leukemia who are ineligible for high-dose chemotherapy.
