Pfizer Is Pursuing Oncology, Inflammation, and Immunology Research
On May 9, 2017, the US Food and Drug Administration (or FDA) approved Pfizer (PFE) and Merck’s Bavencio (avelumab) as a treatment option for patients suffering from locally advanced or metastatic urothelial carcinoma (or UC).
Jan. 4 2018, Updated 10:34 a.m. ET
Recent FDA approvals for Bavencio
On May 9, 2017, the US Food and Drug Administration (or FDA) approved Pfizer (PFE) and Merck’s Bavencio (avelumab) as a treatment option for patients suffering with locally advanced or metastatic urothelial carcinoma (or UC) and witness disease progression during or after being previously treated with platinum chemotherapy or within a year of neoadjuvant or adjuvant platinum-containing chemotherapy.
On December 21, 2017, the FDA also granted breakthrough therapy designation to avelumab-Inlyta (axitinib) combination therapy in first-line advanced renal cell carcinoma indication. Notably, Pfizer accounts for around 0.80% of the Vanguard Total Stock Market ETF’s (VTI) total portfolio holdings.
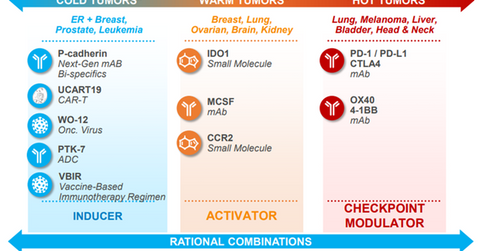
Oncology research programs
Pfizer and Merck are currently exploring Bavencio in combination with 4-1BB agent in patients not previously treated with the checkpoint inhibitor. Data from this non-pivotal trial is anticipated in 2018. Pfizer also expects data from a trial evaluating a combination of Bavencio, 4-1BB agent, and OX40 monoclonal antibody in the later part of 2018. Additionally, Pfizer is also involved in developing a vaccine against prostate cancer. The robust research pipeline could help Pfizer pose tough competition to other major oncology players such as Merck (MRK), Roche Holdings (RHHBY), and Bristol-Myers Squibb (BMY) in future years.
Inflammation and immunology research programs
On December 14, 2017, Pfizer initiated a phase three program to study the efficacy and safety of investigational Janus kinase 1 (or JAK1) inhibitor, PF-04965842, in moderate-to-severe atopic dermatitis (or AD) indication. Based on data presented by the company in the EADV conference in Europe, this once-daily oral therapy has shown clearing or almost clearing of skin lesions in almost 45% of the AD patients.
