
Fact Check: Do Retail Investors Own 90% of AMC like Adam Aron Says?
How has the institutional ownership of AMC changed recently and should you follow the smart money, as institutional funds are known?
Track the hottest index funds and check in on the performance of mutal funds with Market Realist's financial analysts.




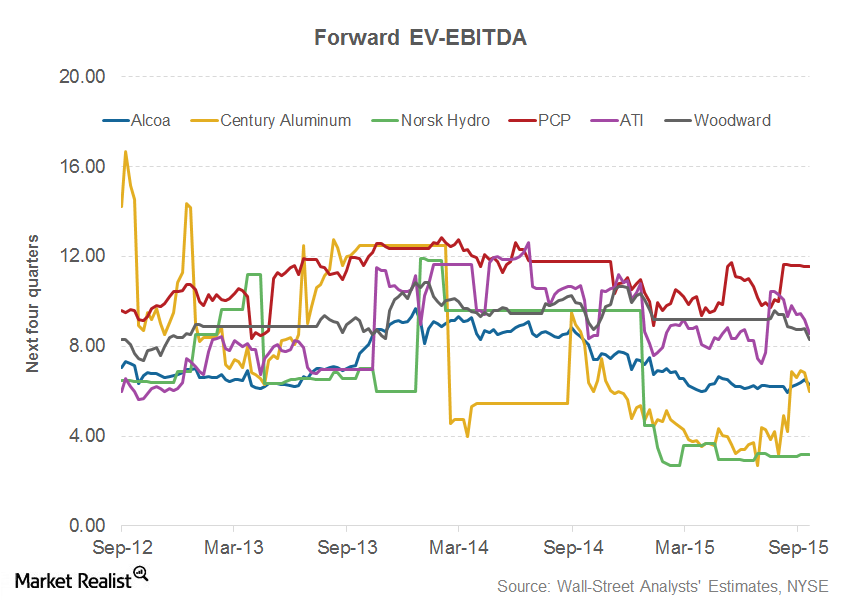

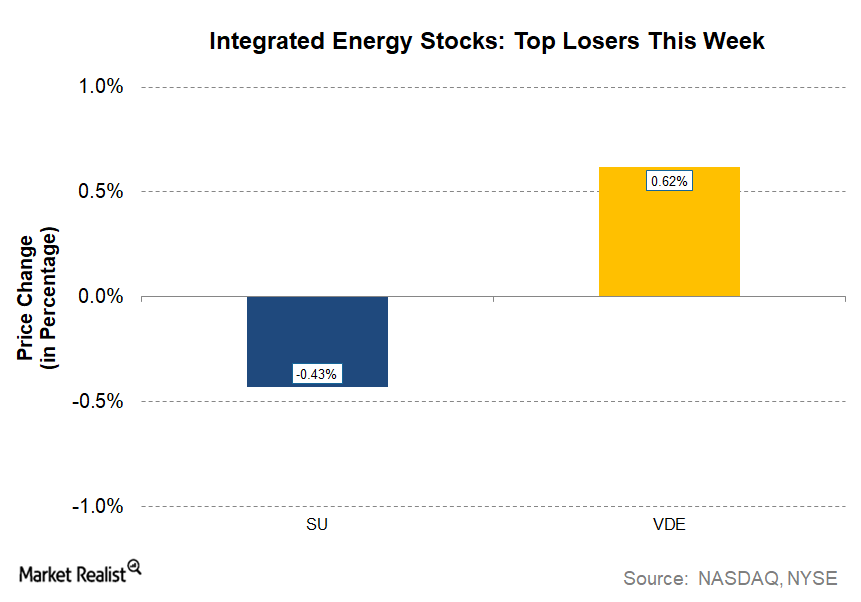
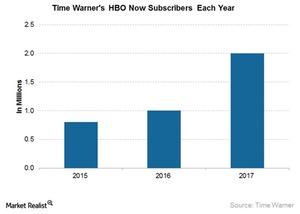
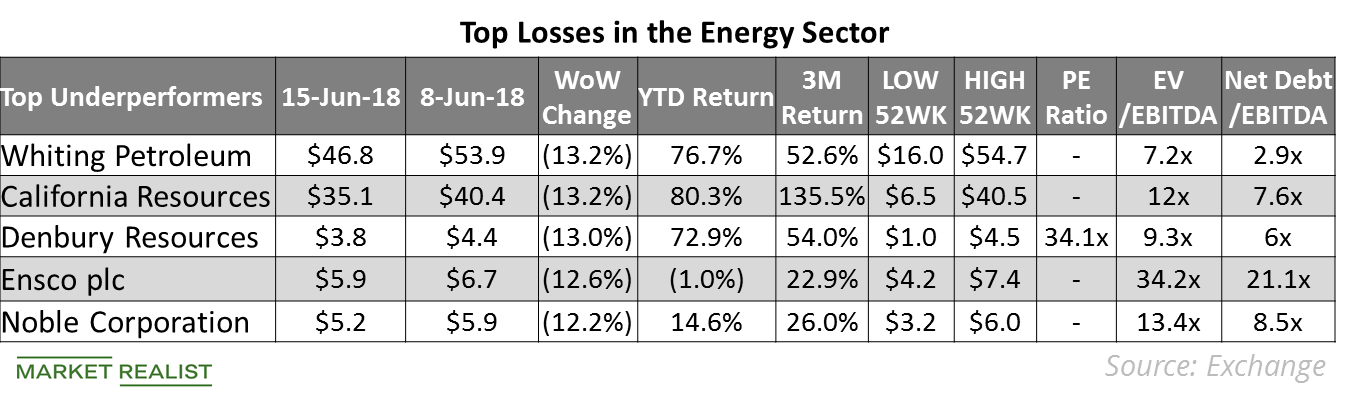
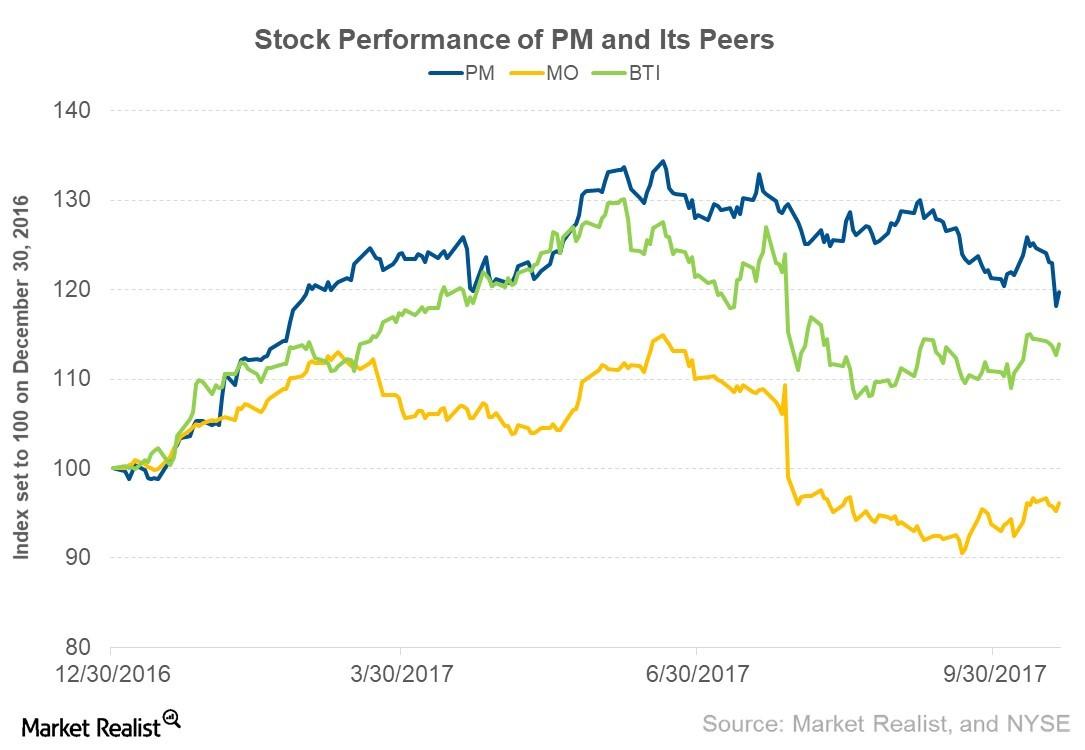
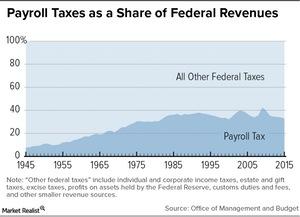
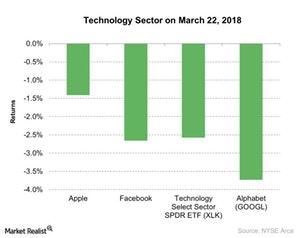
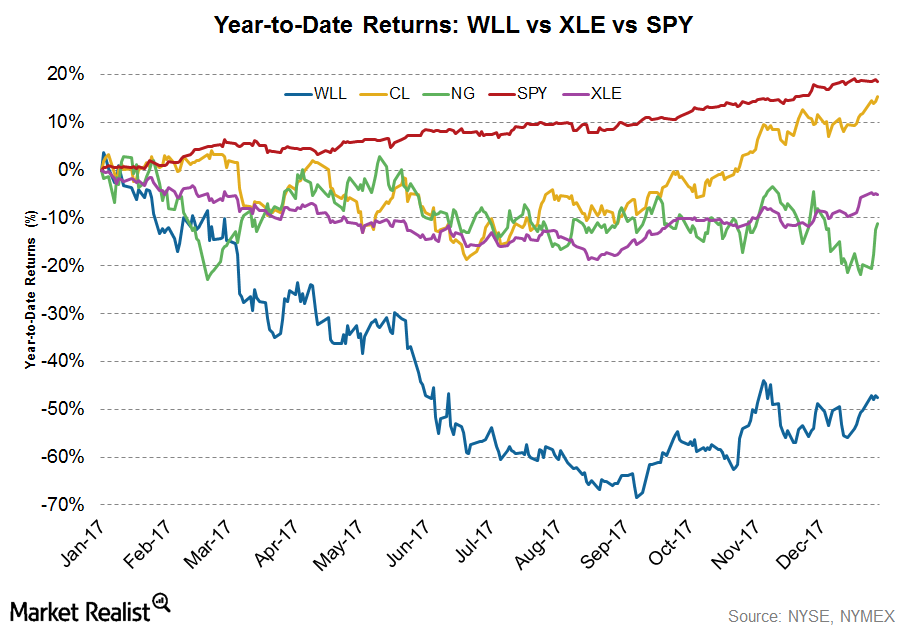
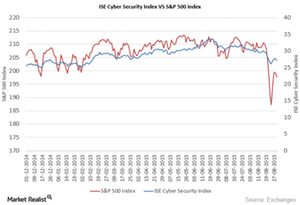
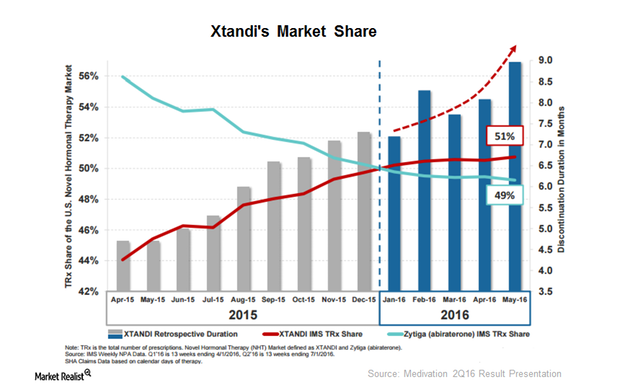
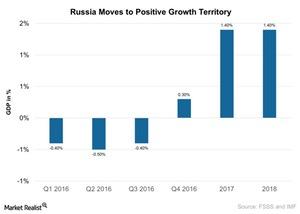
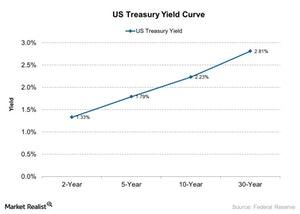
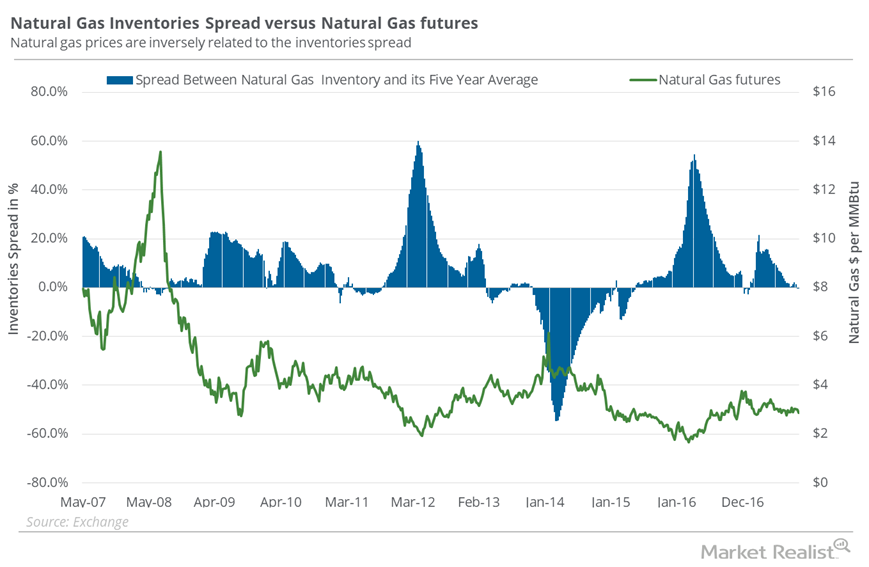

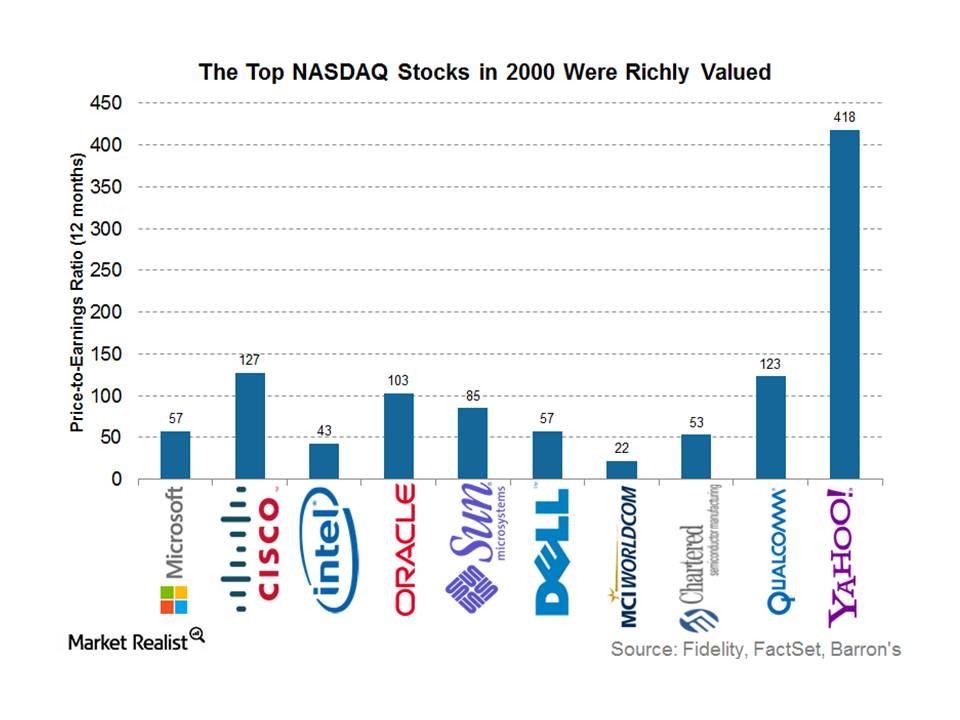

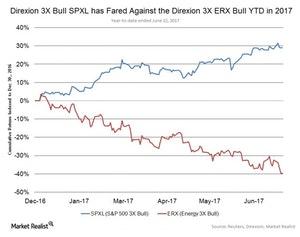
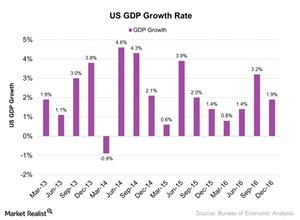


© Copyright 2026 Market Realist. Market Realist is a registered trademark. All Rights Reserved. People may receive compensation for some links to products and services on this website. Offers may be subject to change without notice.