Restoration Hardware Holdings Inc
Latest Restoration Hardware Holdings Inc News and Updates
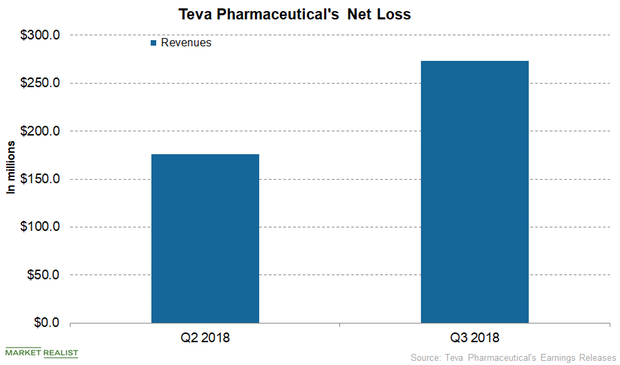
Teva Pharmaceutical: Earnings Trends and Recent Developments
Teva Pharmaceutical’s net income and diluted EPS in the first nine months of 2018 amounted to $541.0 million and $0.53, respectively.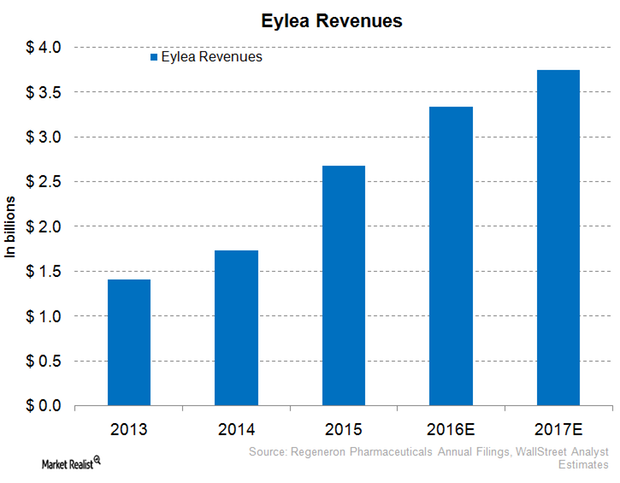
Why Eylea Could Face Tough Competition in 2016
In 2016, Regeneron expects to face increased competition from Roche Holding’s Lucentis (Ranibizumab) and Avastin (Bevacizumab) for Eylea.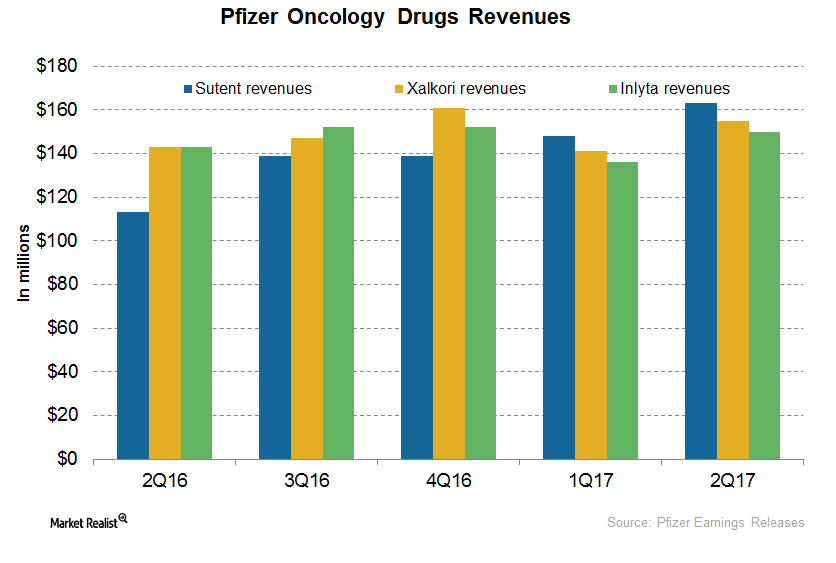
A Post-2Q17 Update on Pfizer’s Oncology Drugs: Sutent, Xalkori, and Inlyta
In 2Q17, Inlyta generated revenues of ~$88 million, which represents an ~19% decline on a YoY basis and ~4% growth on a quarter-over-quarter basis.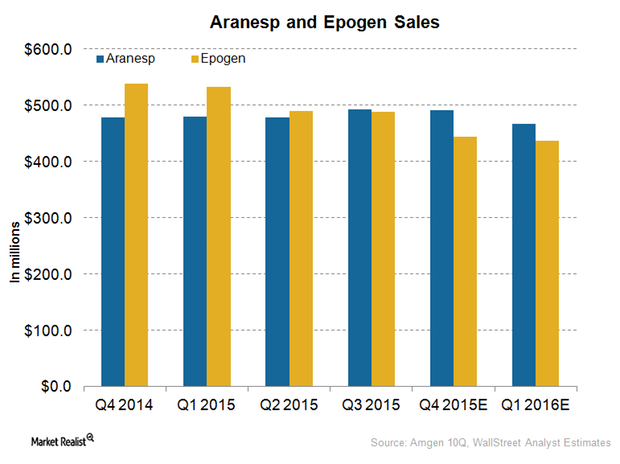
Amgen’s Nephrology Drugs Expect Falling Revenue in 4Q15
Analysts projected a fall in the revenue for Amgen’s nephrology drugs, Aranesp and Epogen, in 4Q15. The drugs are expected to suffer in the coming quarters.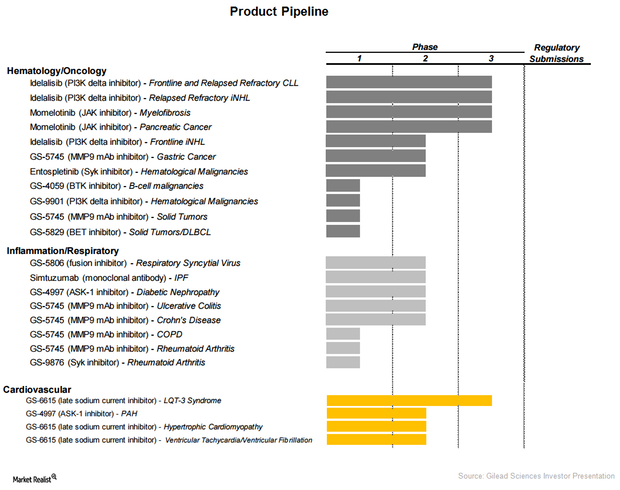
Gilead Sciences’ Product Line Extension
As part of its significant product line extension, Gilead Sciences (GILD) is entering therapeutic areas such as oncology, pulmonology, and cardiology.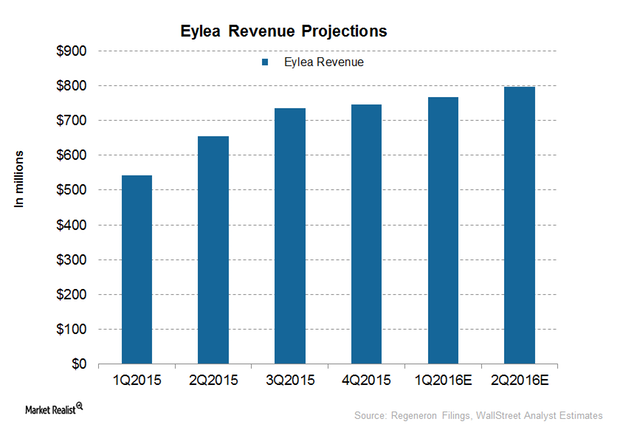
Worst-Case Scenario for Regeneron: What If Eylea Sales Slow Down by 2018?
Regeneron Pharmaceuticals (REGN) depends heavily upon its key drug, Eylea, which recorded global sales of $4.1 billion during fiscal 2015.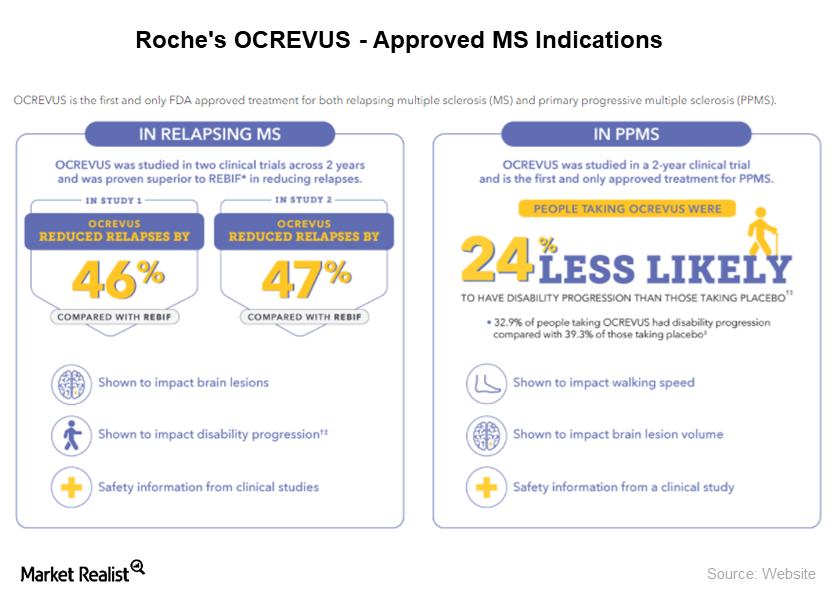
Why NICE Didn’t Approve Roche’s Ocrevus for Multiple Sclerosis
On April 5, 2018, Roche’s (RHHBY) MS (multiple sclerosis) drug Ocrevus was denied recommendation by NICE.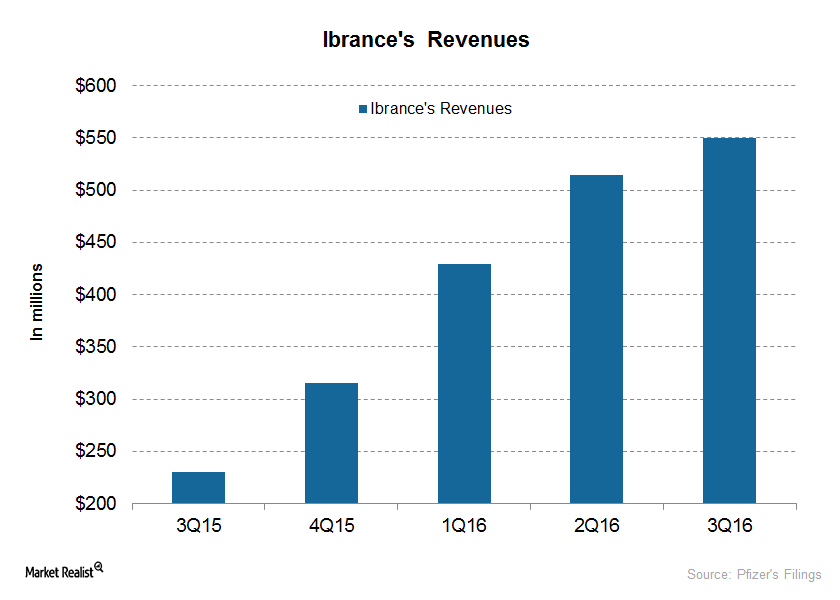
Ibrance Is the Only Registered CDK 4/6 Inhibitor for Breast Cancer
Since its launch in February 2015, Pfizer’s Ibrance has quickly captured the advanced breast cancer market and has reached more than 40,000 patients.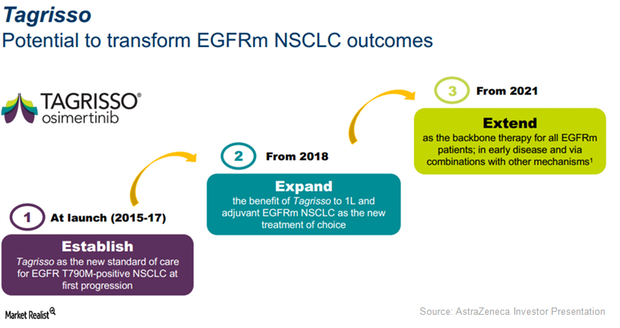
Tagrisso Expected to Be a Key Growth Driver for AstraZeneca in 2017
Launched in Japan in 2Q16, AstraZeneca’s (AZN) 1Q17 revenues for Tagrisso approached $39 million in this major emerging market.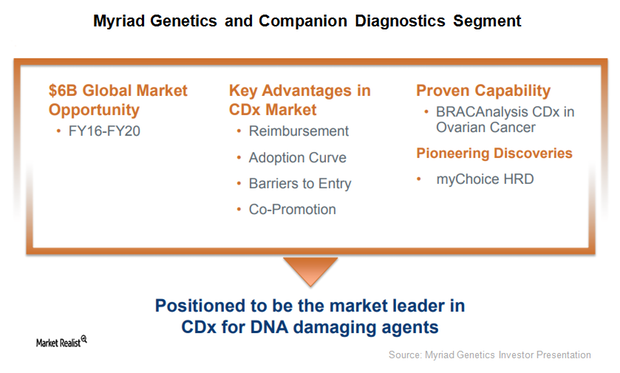
This Could Be a Solid Growth Driver for Myriad Genetics in 2018
Myriad Genetics (MYGN) announced the U.S. Food and Drug Administration’s (or FDA) acceptance of its supplementary premarket approval application for BRACAnalysis CDx, a DNA sequencing companion diagnostic test.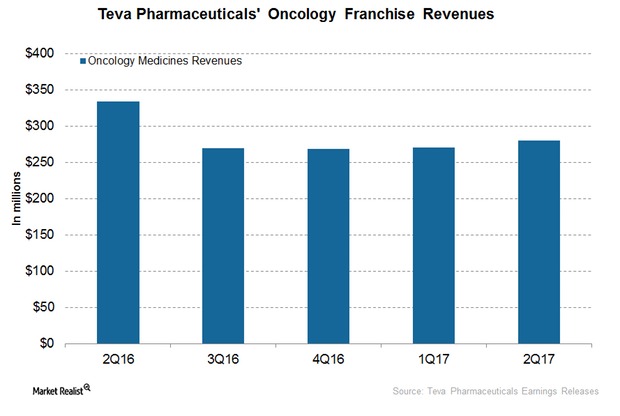
The Latest on TEVA’s Oncology Business
In 1H17, Teva Pharmaceutical’s (TEVA) oncology business generated revenues of ~$550 million, or ~9% lower YoY (year-over-year).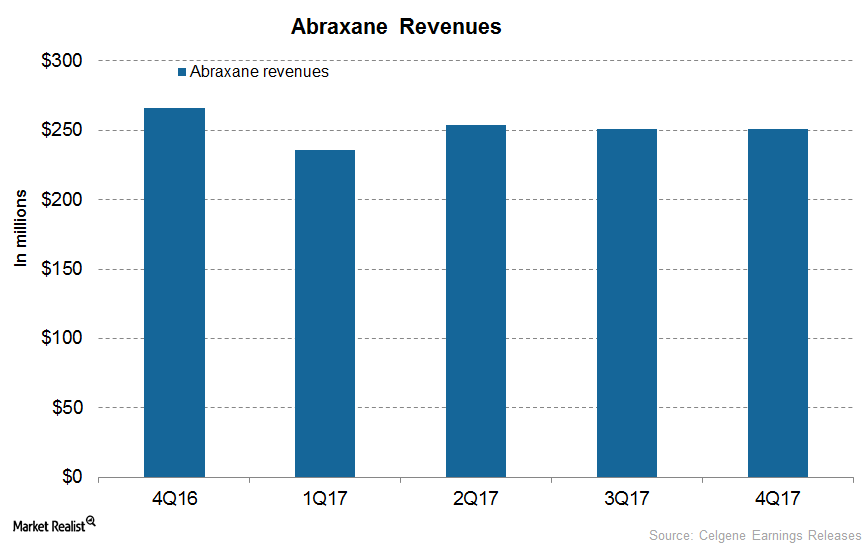
How’s Celgene’s Abraxane Positioned after 4Q17?
In 4Q17, Celgene’s (CELG) Abraxane generated revenues of $251 million, which reflected a decline of ~6% on a YoY (year-over-year) basis.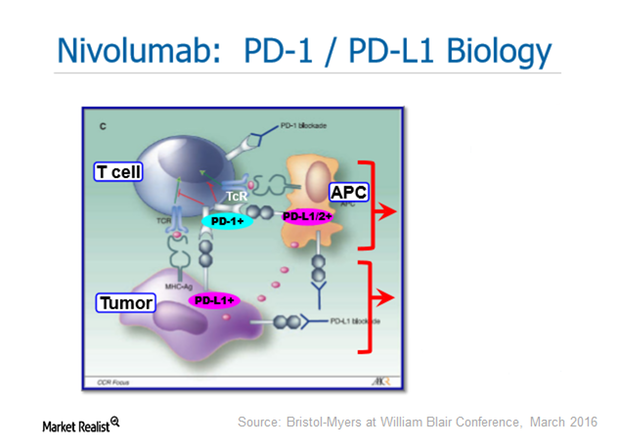
Brace Yourself: AstraZeneca Could Be a Fourth Entrant into the PD-1/PD-L1 Drug Class
The PD-1 (programmed death-1)/PD-L1 (programmed death-ligand 1) class consists of Bristol-Myers Squibb’s Opdivo, Merck’s Keytruda, and Roche’s Tecentriq.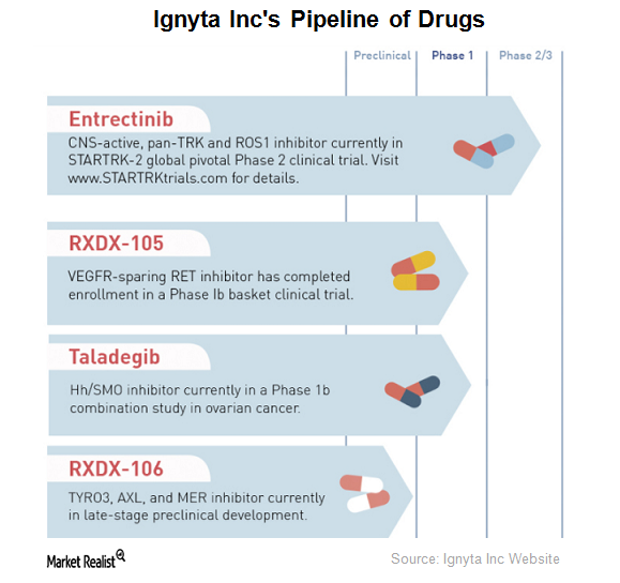
Ignyta’s Drug Pipeline
Ignyta (RXDX) has completed enrollment for a Phase 1 clinical trial of RXDX-105, an orally bioavailable small molecule tyrosine kinase inhibitor.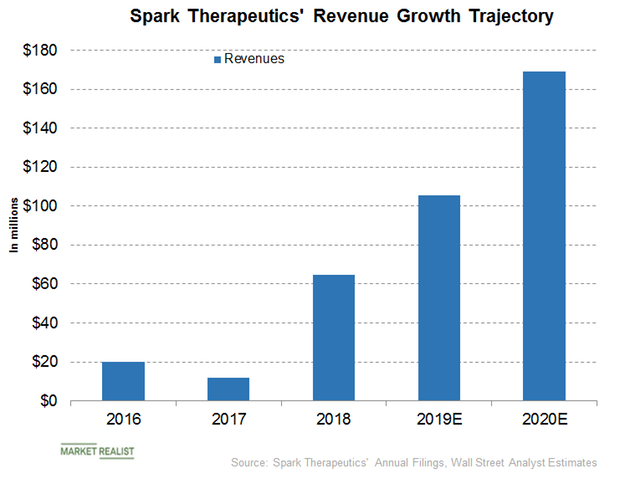
A Look at Spark’s Potential Revenue Contribution to Roche Holdings
Wall Street analysts have projected Spark Therapeutics’ revenues to be $105.64 million, $169.22 million, and $263.76 million for fiscal 2019, fiscal 2020, and fiscal 2021, respectively.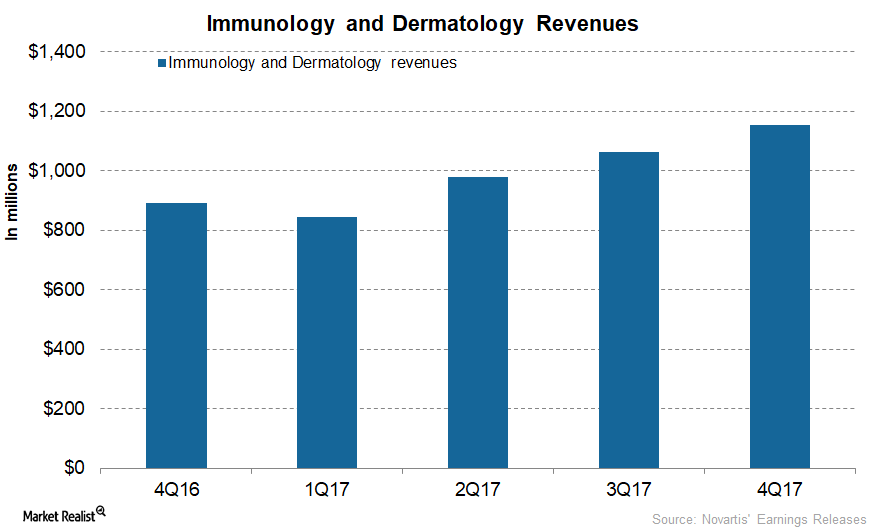
A Look at Novartis’s Immunology and Dermatology Segment’s Performance
In 4Q17, Novartis’s Immunology and Dermatology segment generated revenues of $1.2 billion, ~30% growth on a year-over-year (or YoY) basis and ~9% growth quarter-over-quarter.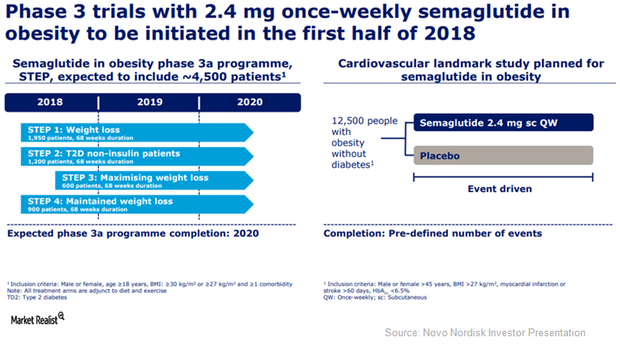
Semaglutide May Prove to Be an Effective Anti-Obesity Therapy
Novo Nordisk plans to initiate its Phase 3a program, STEP, to study the efficacy of 2.4 mg of semaglutide once per week in obesity indications in 1H18.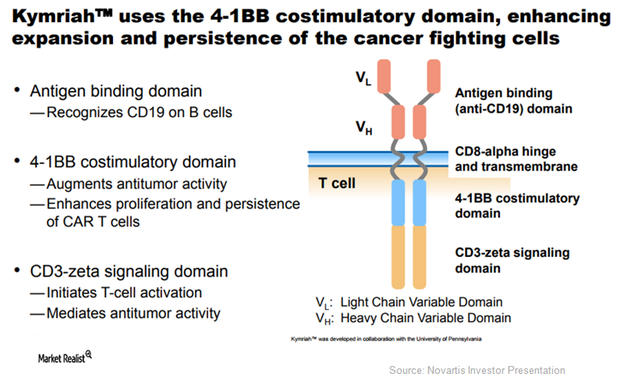
Novartis’ Kymriah: The First Gene Therapy to Be Approved in the US
The National Cancer Institute estimates that the incidence of ALL in patients aged 20 or younger is ~3,100 in the US.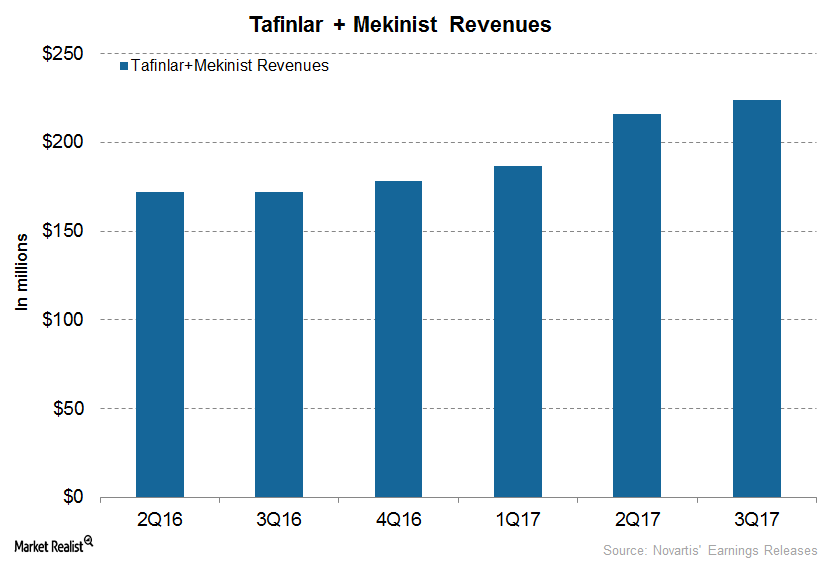
How Is Novartis’s Tafinlar+Mekinist Positioned for 2018?
In 1Q17, 2Q17, and 3Q17, Tafinlar+Mekinist reported revenues of $187 million, $216 million, and $224 million, respectively.
Zelboraf Could Boost Roche’s Sales Growth in 2018
Roche’s (RHHBY) Zelboraf (vemurafenib) is indicated for the treatment of individuals with unresectable or metastatic melanoma with the BRAF V600 mutation.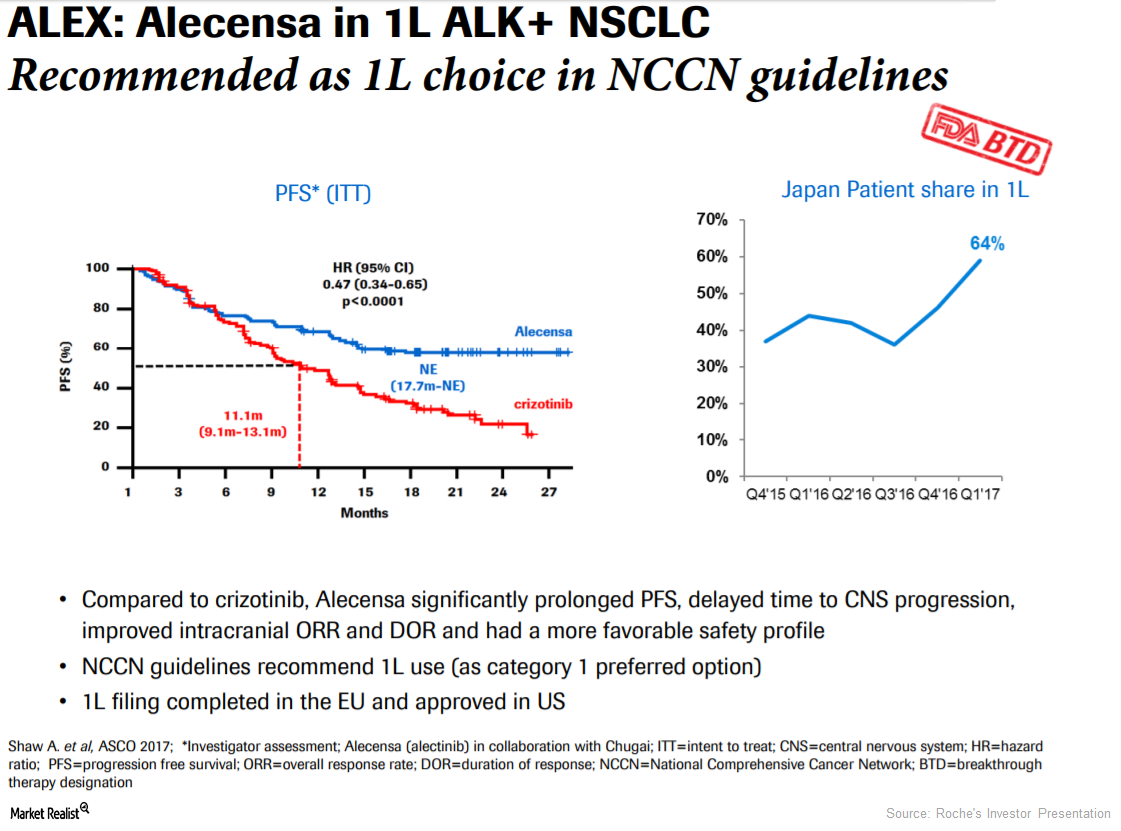
Approval of Alecensa in Europe Could Boost Roche’s Revenue Growth
In 1Q17, 2Q17, and 3Q17, Roche’s Alecensa reported revenues of 68 million Swiss francs, 80 million Swiss francs, and 96 million Swiss francs, respectively.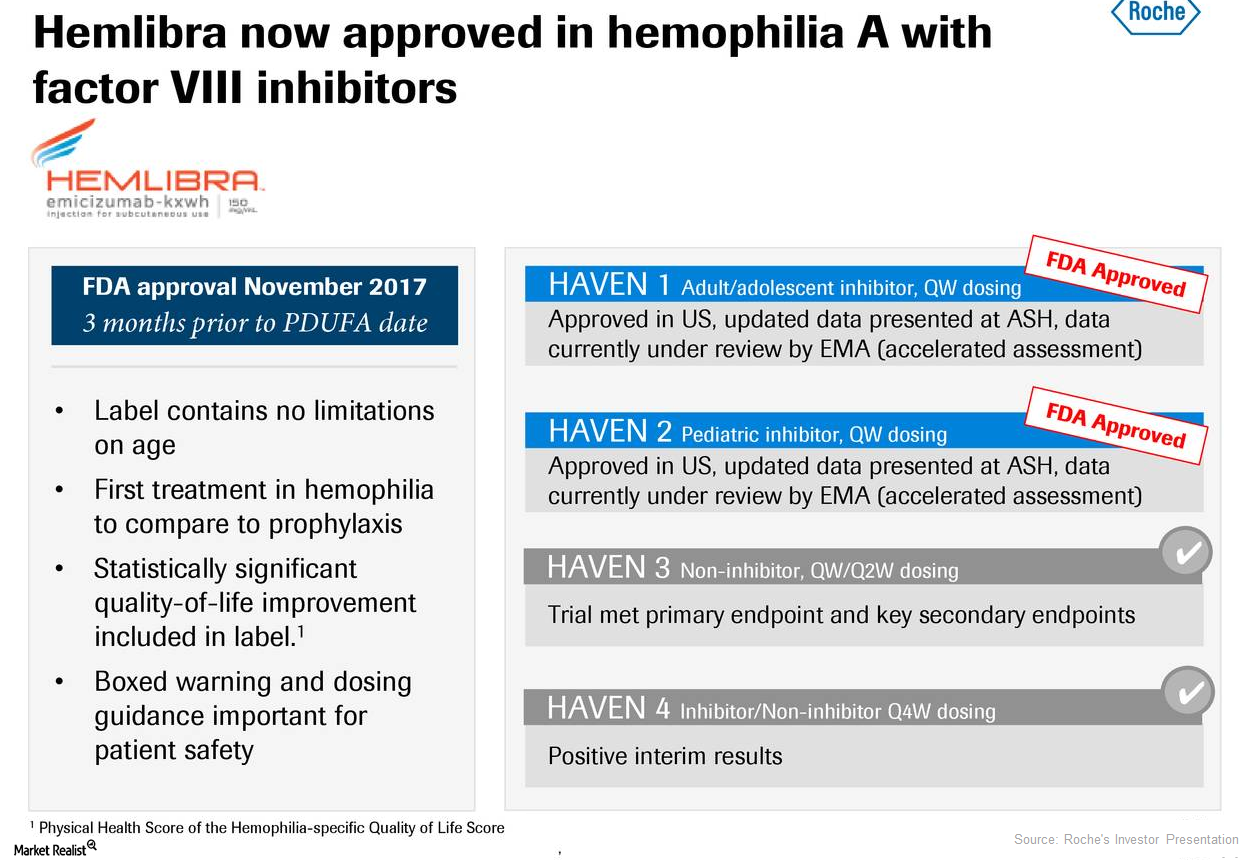
How Is Roche’s Hemlibra Positioned for 2018?
Roche’s (RHHBY) Hemlibra is used for the prevention and reduction of the frequency of bleeding episodes in individuals with hemophilia A with factor VIII inhibitors.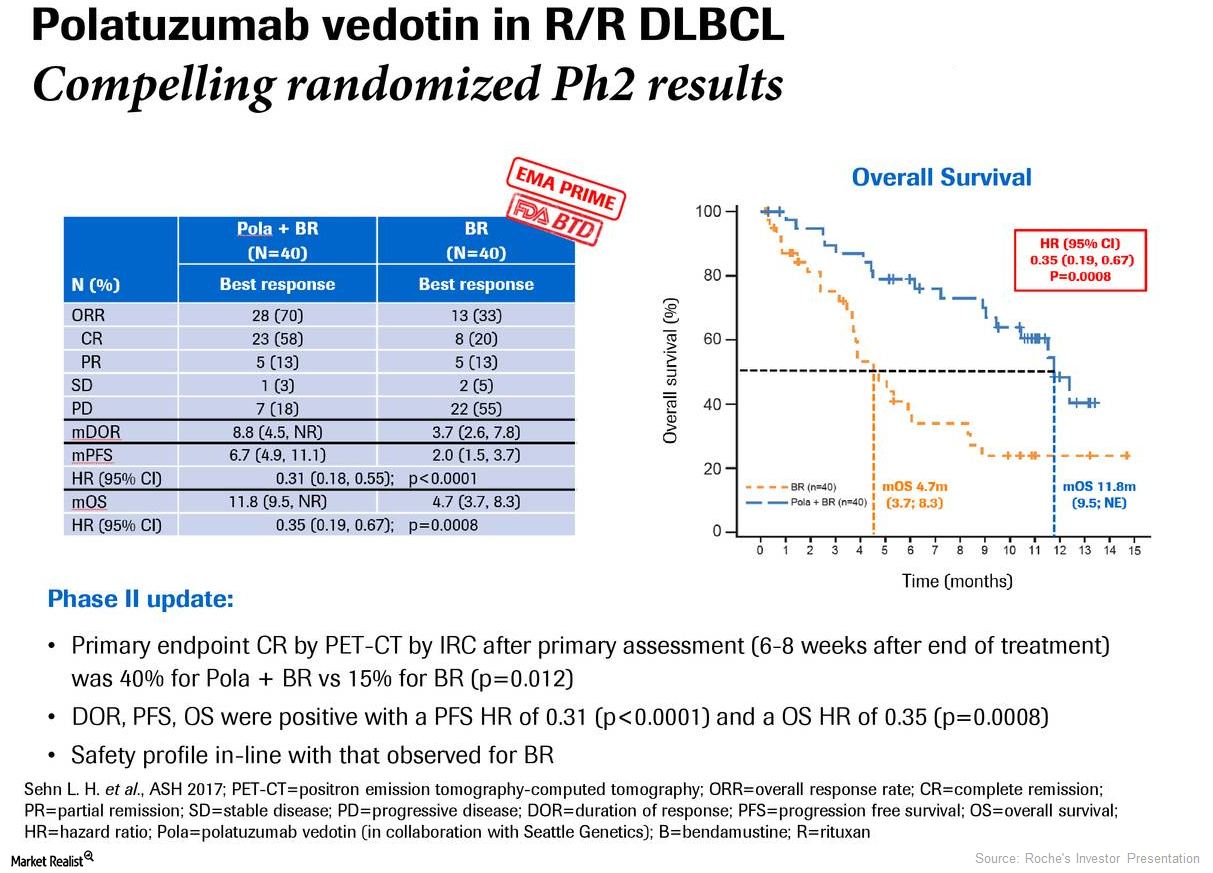
What to Expect from Roche’s Investigational Drug Polatuzumab Vedotin
In December 2017, Roche (RHHBY) presented the results of its randomized phase two GO29365 trial.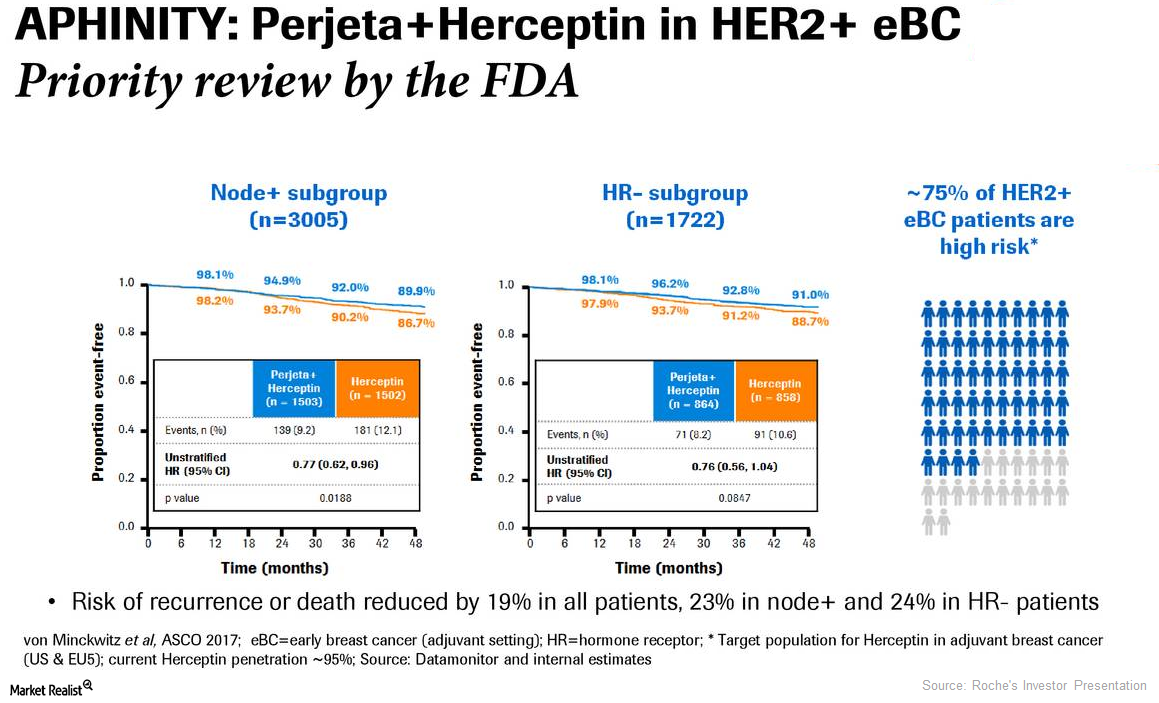
Perjeta Could Significantly Boost Roche’s Revenue Growth in 2018
In December 2017, the Food and Drug Administration approved Roche’s (RHHBY) Perjeta based on the results of its phase three Aphinity trial.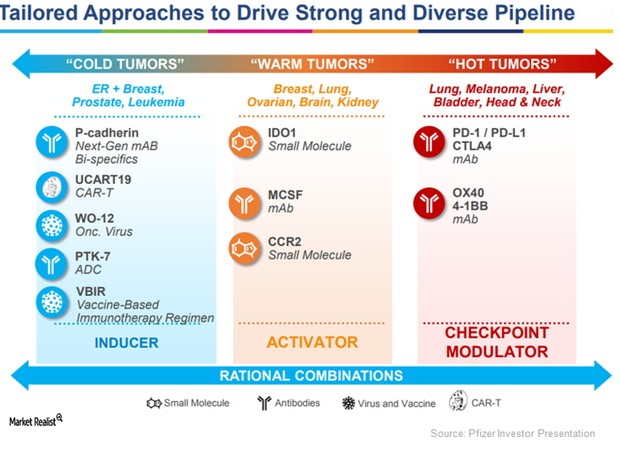
Pfizer Is Pursuing Oncology, Inflammation, and Immunology Research
On May 9, 2017, the US Food and Drug Administration (or FDA) approved Pfizer (PFE) and Merck’s Bavencio (avelumab) as a treatment option for patients suffering from locally advanced or metastatic urothelial carcinoma (or UC).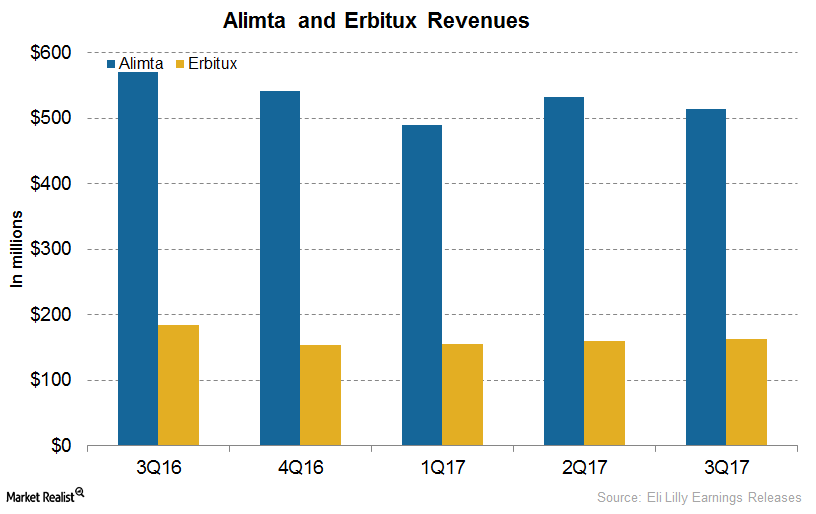
An Update on Eli Lilly’s Oncology Drugs: Alimta, Erbitux, and Gemzar
In 3Q17, Eli Lilly’s (LLY) Alimta generated revenues of $514.5 million, a ~10% increase on a year-over-year (or YoY) basis and a 3% decline on a quarter-over-quarter basis.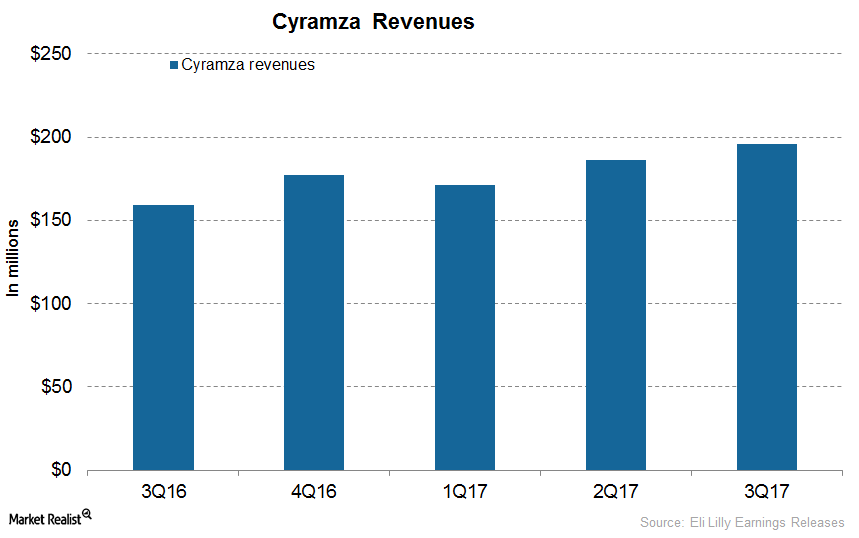
How Has Eli Lilly’s Cyramza Performed
In 3Q17, Eli Lilly’s (LLY) Cyramza generated revenues of $196 million, which reflected ~23% growth on a YoY basis and 5% growth on a quarter-over-quarter basis.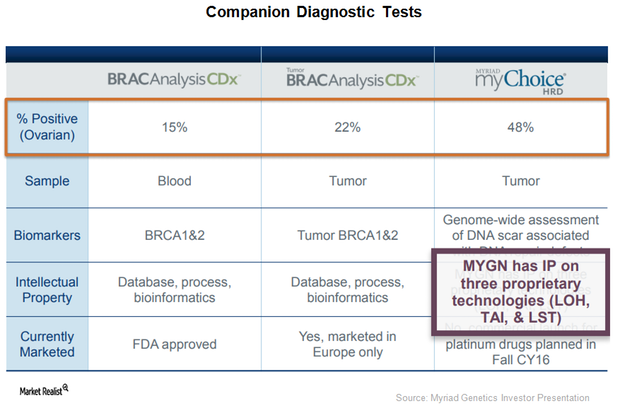
BRACAnalysis CDx Received FDA Approval for Ovarian Cancer Indication
On March 27, 2017, the FDA also approved BRACAnalysis CDX test as a complementary diagnostic test to be used with ovarian cancer maintenance therapy Tesaro’s (TSRO) Zejula (miraparib).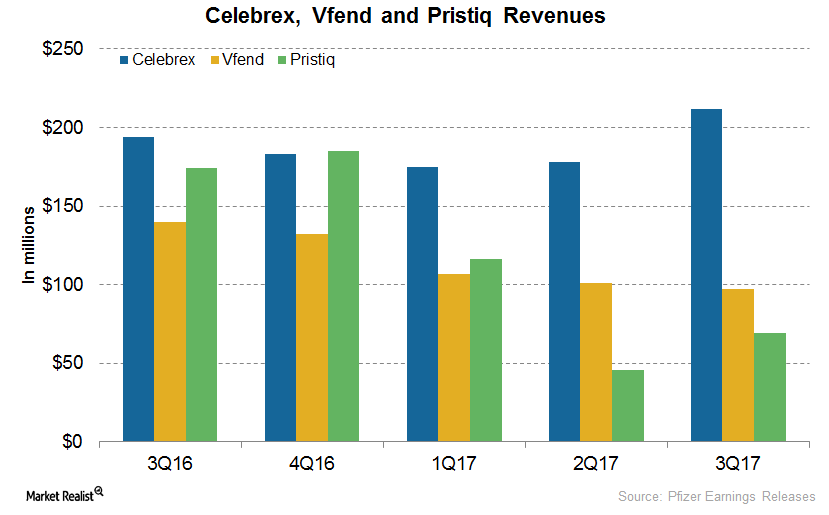
A Look at How These Pfizer Drugs Have Performed in 2017
In 3Q17, Pfizer’s (PFE) Celebrex generated revenues of $212 million, a ~9% increase on a year-over-year (or YoY) basis and a 19% increase on a quarter-over-quarter basis.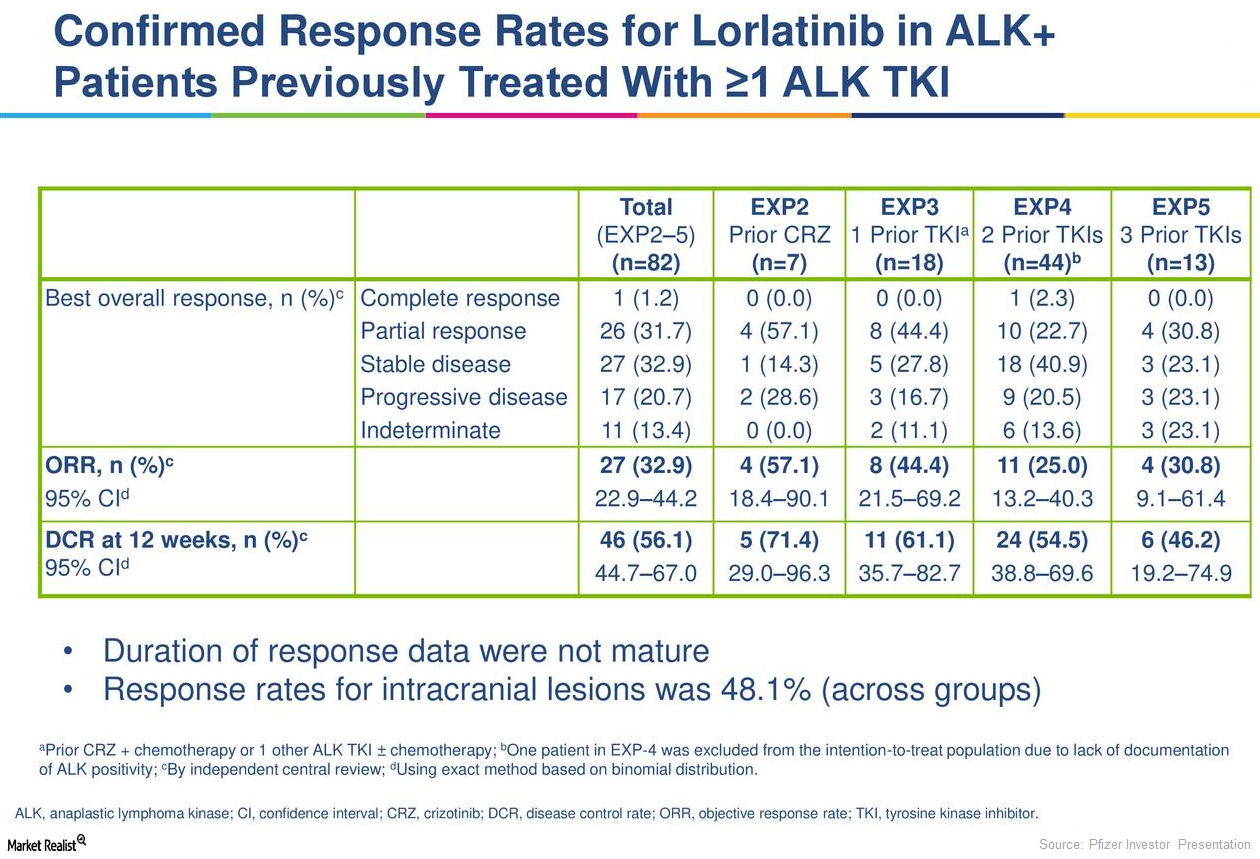
Could Lorlatinib Be a Long-Term Growth Driver for Pfizer?
Lorlatinib is Pfizer’s (PFE) investigational next-generation ALK/ROS-1 tyrosine kinase inhibitor in clinical trials to evaluate its safety and efficacy in the treatment of ALK-positive metastatic non-small cell lung cancer.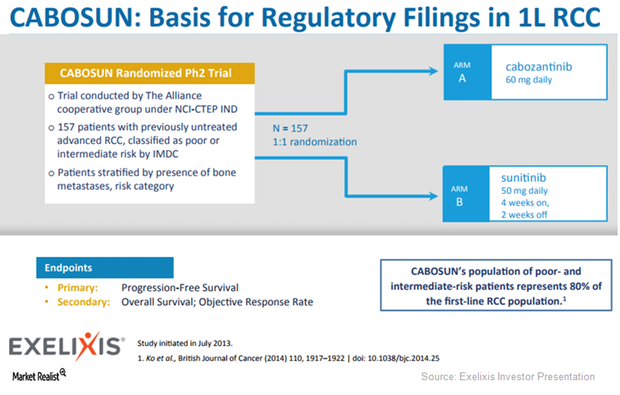
Behind Exelixis’s Cabometyx Strategy for 2018
Exelixis (EXEL) expects the FDA’s approval for Cabometyx for first-line RCC (renal cell carcinoma) to be a major revenue driver.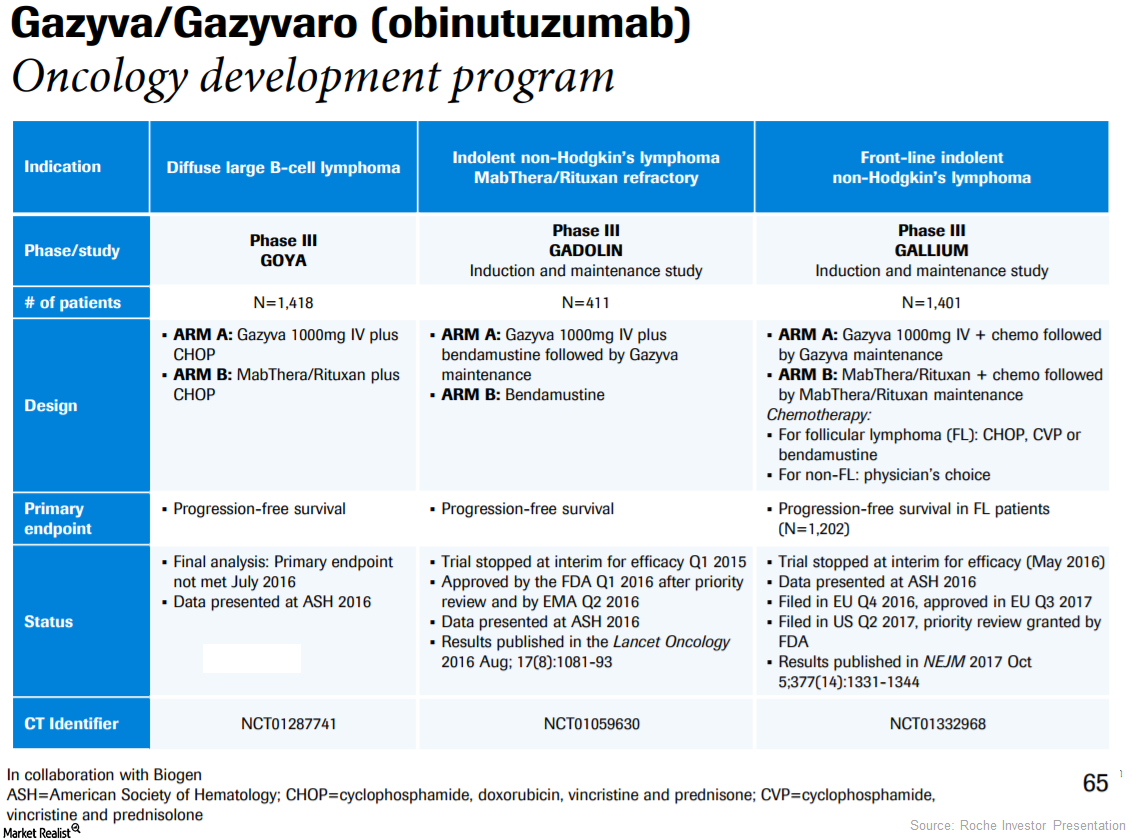
How Roche’s Oncology Drug Gazyva Is Positioned after 3Q17
In 3Q17, Roche’s (RHHBY) Gazyva generated revenues of 69 million Swiss francs, which reflected ~34% growth on a year-over-year (or YoY) basis.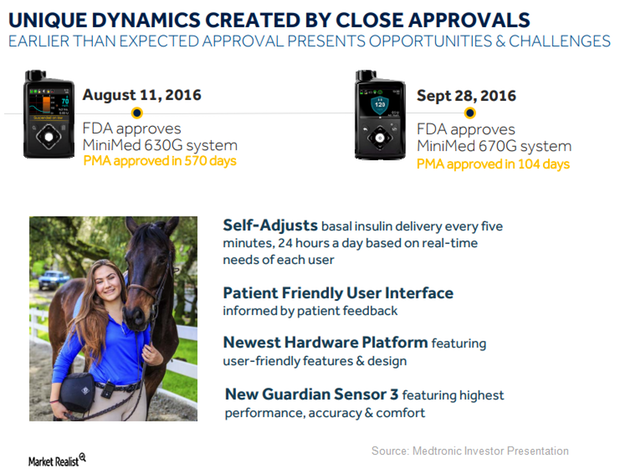
Medtronic Saw Several Challenges during Launch of MiniMed 670G
In fiscal 2H17, Medtronic launched the priority access program to first target those patients who were interested in purchasing MiniMed 670G.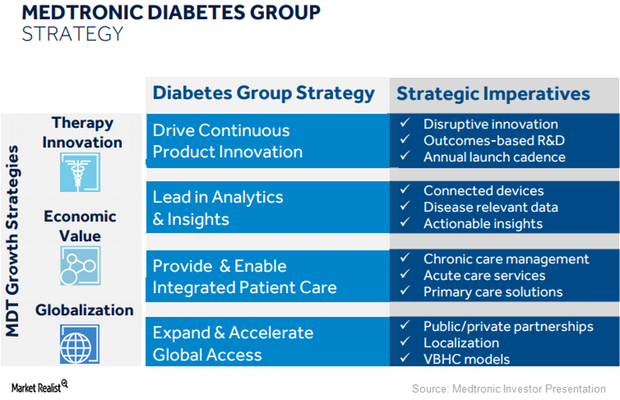
What’s Medtronic’s Long-Term Growth Strategy for Its Diabetes Business?
Medtronic (MDT) expects its diabetes business to witness a temporary sequential drop in revenues in 2Q18 and then return back to growth in the second half of fiscal 2018.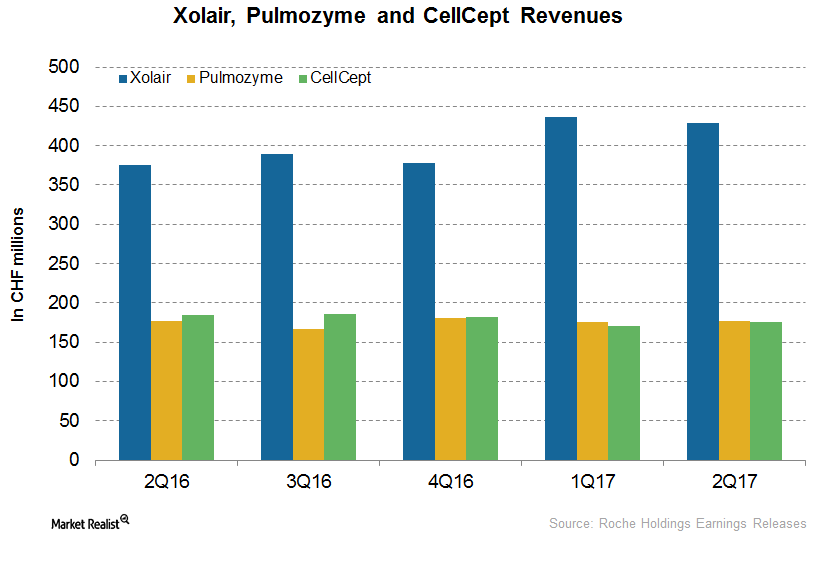
Roche’s Immunology Drugs Xolair, Pulmozyme, and CellCept
In the first half of 2017, Roche’s (RHHBY) Xolair reported revenues of CHF 866.0 million, which is a 17.0% rise on a YoY (year-over-year) basis.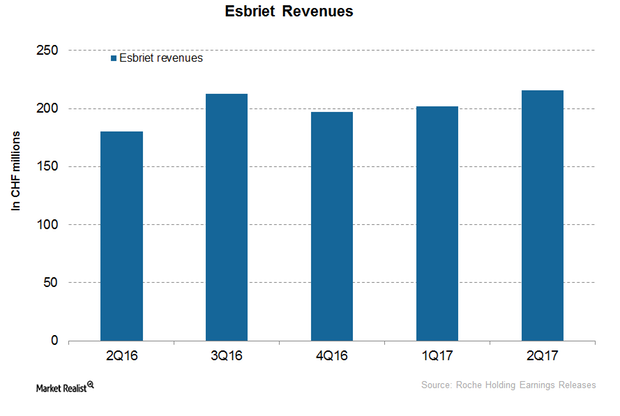
Esbriet Could Boost Roche’s Revenue Growth in 2H17
In the first half of 2017, Roche’s (RHHBY) Esbriet reported revenues of CHF 418.0 million, which reflected a ~16.0% growth on a YoY basis.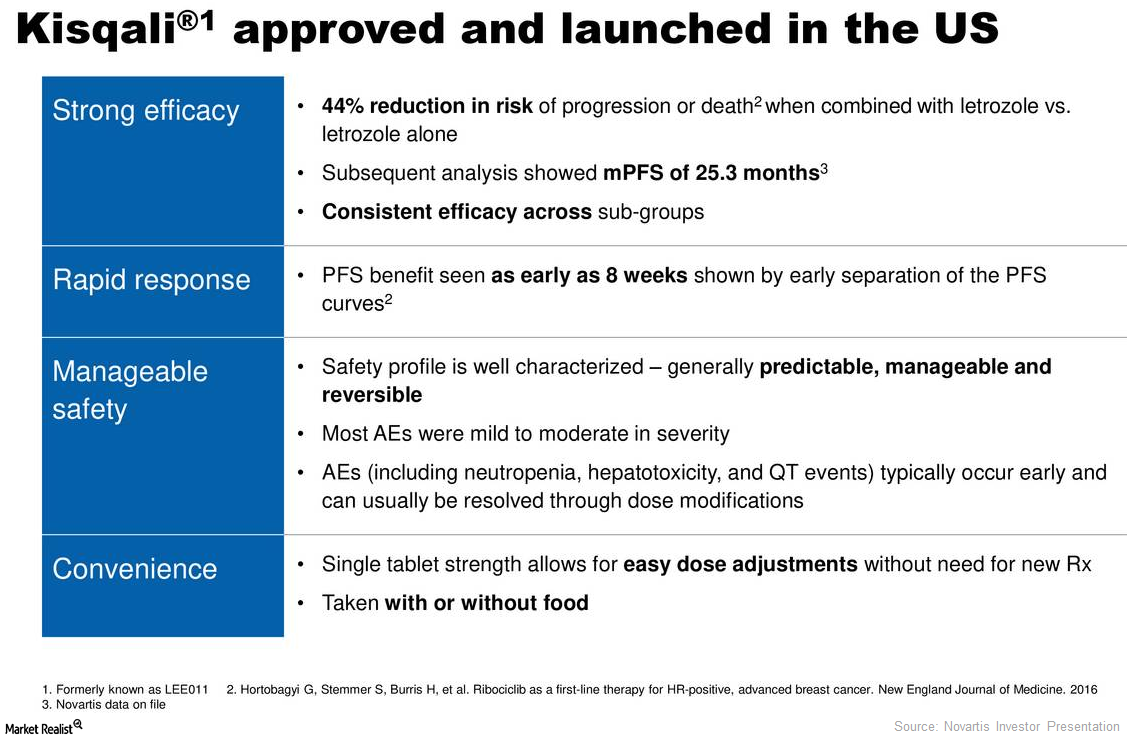
Kisqali Could Significantly Boost Novartis’s Revenue Growth
In 1H17 and 2Q17, Novartis’s (NVS) Kisqali reported revenues of around $15 million and $8 million, respectively.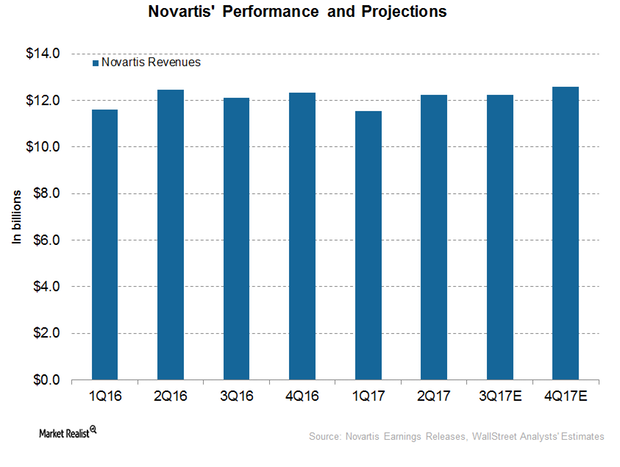
How Did Novartis Perform in 1H17?
In 1H17, Novartis (NVS) reported revenues of around $23.8 billion, a ~1% decline on a year-over-year (or YoY) basis.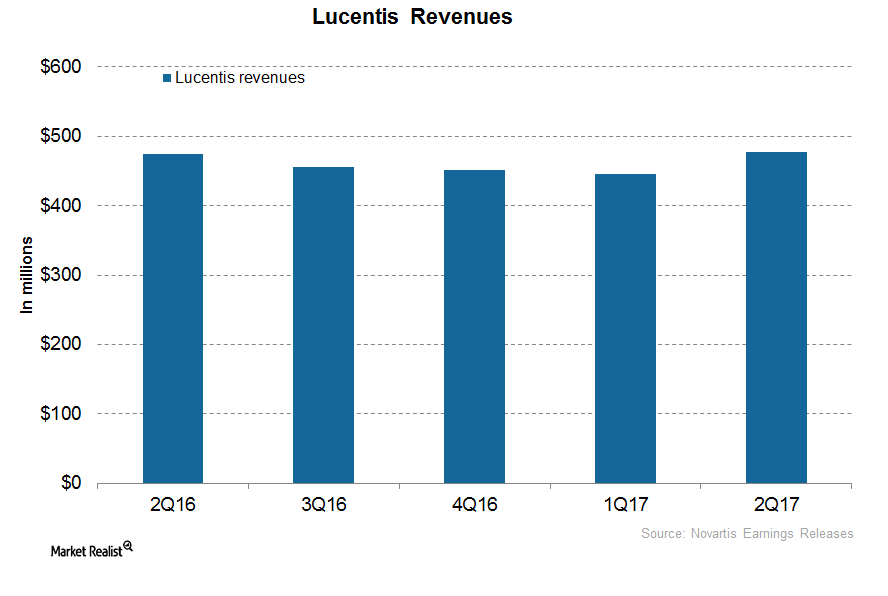
How Is Novartis’s Lucentis Positioned after 1H17?
In 1H17, Novartis’s (NVS) Lucentis reported revenues of around $922 million, which is a ~1% decline on a year-over-year (or YoY) basis.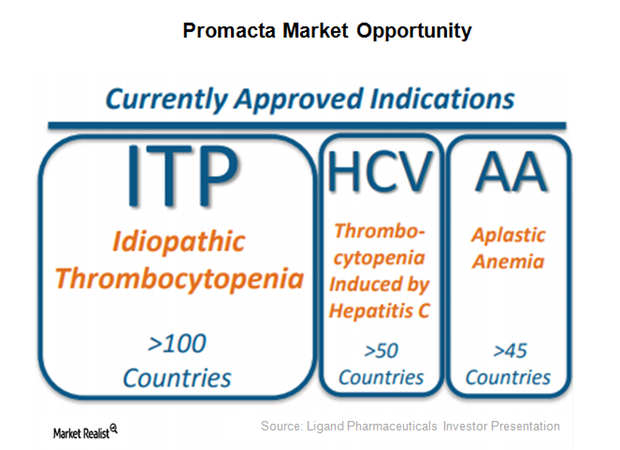
Promacta: A Major Growth Driver for Ligand Pharmaceuticals in 2017
Promacta was discovered by Ligand Pharmaceuticals (LGND) and GlaxoSmithKline (GSK) as a part of their thrombopoietin (or TPO) receptor agonist research collaboration.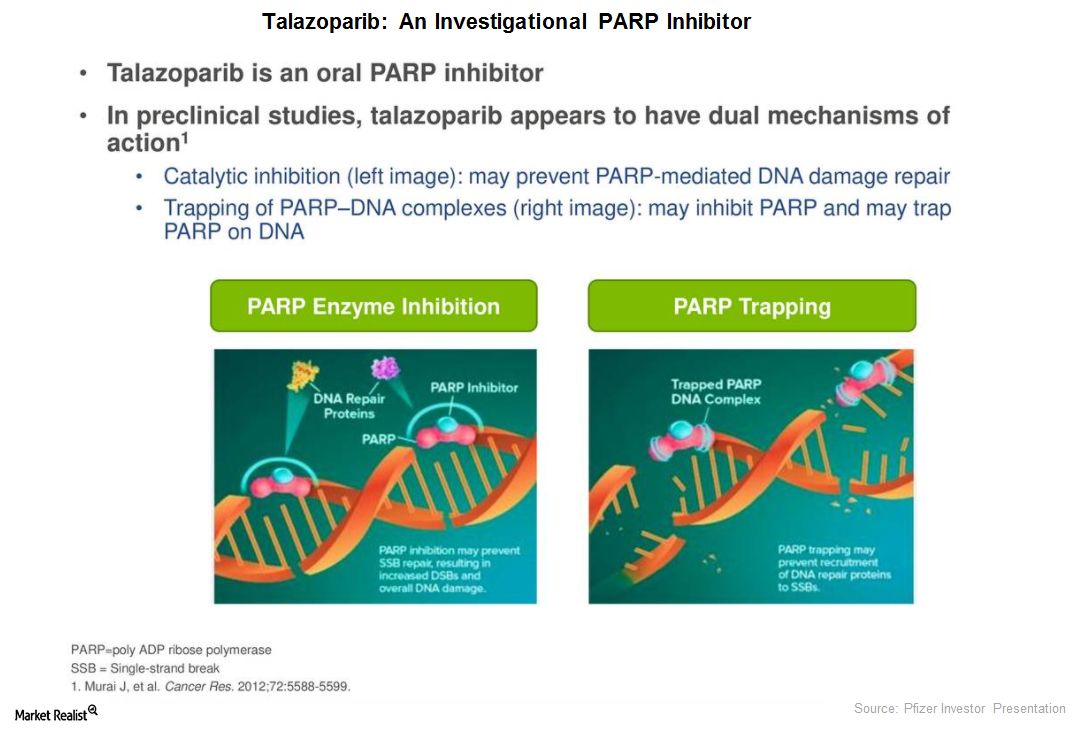
Talazoparib Could Be a Significant Long-Term Growth Driver for Pfizer
In June 2017, Pfizer (PFE) presented the results from the Phase 2 ABRAZO trial.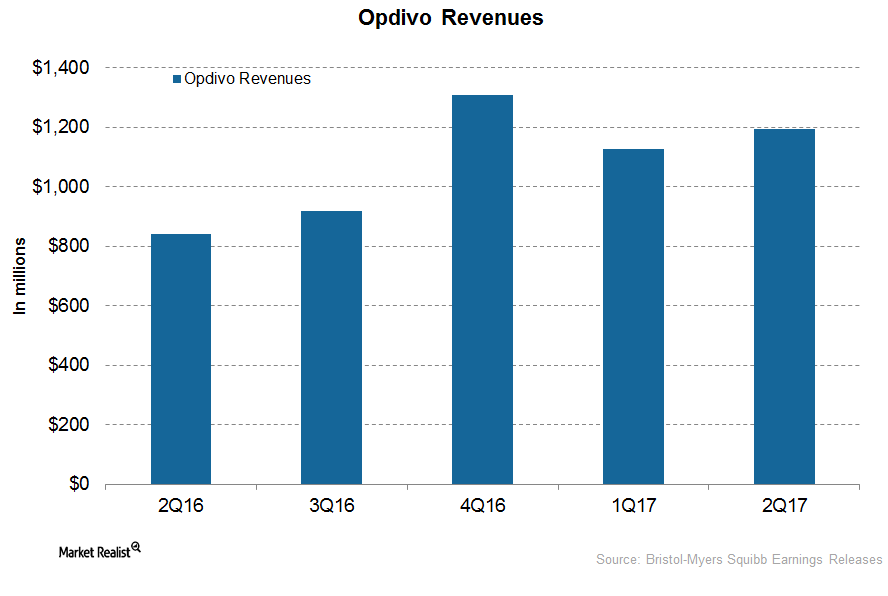
Opdivo Could Drive Bristol-Myers Squibb’s Revenue Growth in 2017
In 2Q17, Bristol-Myers Squibb’s (BMY) Opdivo generated revenues of around $1.2 billion, which reflected ~45% growth on a year-over-year basis.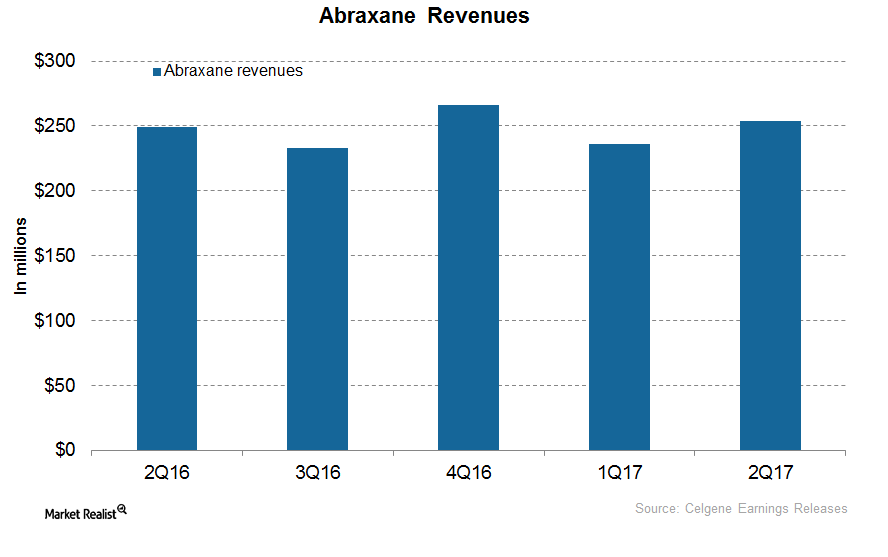
Celgene’s Abraxane Continued Steady Growth in 2Q17
In 2Q17, Celgene’s (CELG) Abraxane generated revenues of around $254 million, which reflected ~2% growth on a year-over-year basis.
An Update on AbbVie’s Clinical Pipeline in 2017
In December 2016, the European Commission granted Venclyxto conditional marketing approval for the treatment of CLL in the presence of 17p deletion or TP53 mutation.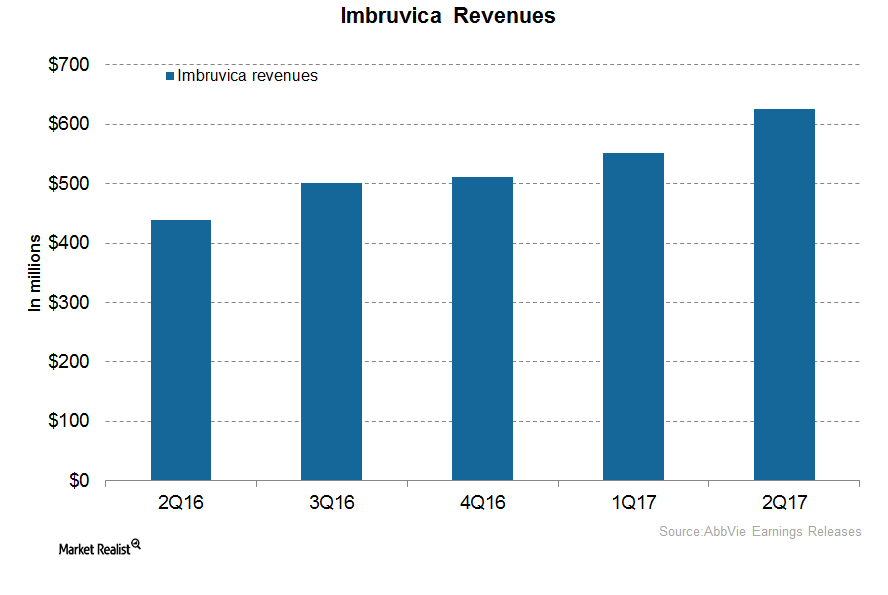
Imbruvica Could Significantly Drive AbbVie’s Revenue Growth
In 2Q17, AbbVie’s (ABBV) Imbruvica generated revenues of around $626.0 million, which reflected a ~43.0% rise YoY and a ~14.0% rise QoQ.
GlaxoSmithKline’s 2Q17 Earnings: Vaccines Business
GSK’s Vaccines business reported 16.0% growth to ~1.1 billion pounds in 2Q17, including 5.0% growth at constant exchange rates and 11.0% positive impact of foreign exchange.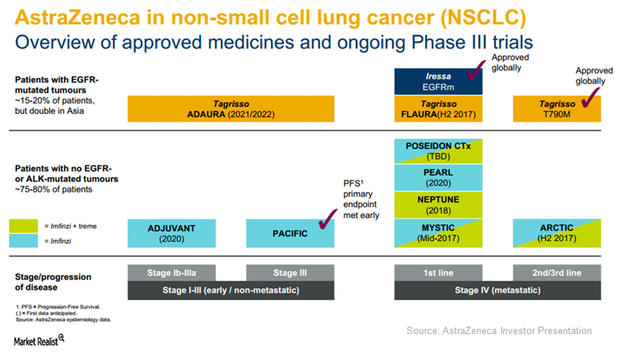
AstraZeneca Aims to Offer Multiple Therapies in NSCLC Segment
EGFR mutant NSCLC has become a major market opportunity for AstraZeneca’s drugs Tagrisso and Iressa.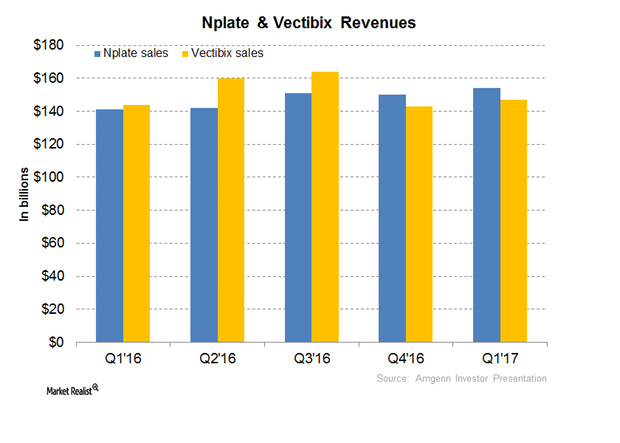
Nplate and Vectibix Could Contribute to Amgen’s Revenue Growth in 2017
In 1Q17, Amgen’s (AMGN) Nplate generated revenues of ~$154 million, which reflected a year-over-year growth rate of 9%.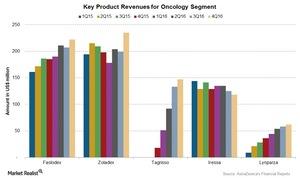
Growth of AstraZeneca’s Oncology Segment in 2016
The revenues for AstraZeneca’s (AZN) Oncology segment rose ~20% at constant exchange rates in 2016.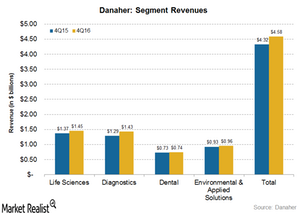
How Did Danaher’s Operating Segments Fare in 4Q16?
Currently, Danaher (DHR) reports its revenue under four operating segments: Life Sciences, Diagnostics, Dental, and Environmental & Applied Solutions.
