Could Lorlatinib Be a Long-Term Growth Driver for Pfizer?
Lorlatinib is Pfizer’s (PFE) investigational next-generation ALK/ROS-1 tyrosine kinase inhibitor in clinical trials to evaluate its safety and efficacy in the treatment of ALK-positive metastatic non-small cell lung cancer.
Nov. 29 2017, Updated 7:35 a.m. ET
About Lorlatinib
Lorlatinib is Pfizer’s (PFE) investigational next-generation ALK/ROS-1 tyrosine kinase inhibitor in clinical trials to evaluate its safety and efficacy in the treatment of ALK-positive metastatic non-small cell lung cancer (or NSCLC). In April 2017, the US Food and Drug Administration (or FDA) granted lorlatinib its “Breakthrough Therapy” designation for the treatment of individuals with ALK-positive metastatic NSCLC who previously underwent treatment with one or more ALK inhibitors.
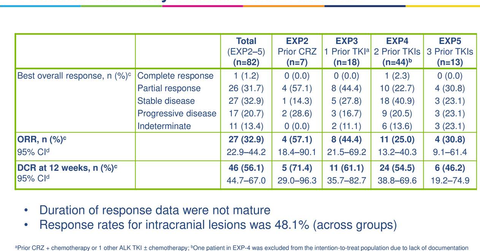
Phase 2 clinical trial results
In October 2017, Pfizer presented results from the phase 2 clinical trials, which evaluated safety and efficacy of lorlatinib in individuals with ALK-positive and ROS1-positive metastatic NSCLC who previously underwent heavy treatment.
The phase 2 clinical trial was conducted on 275 patients with or without asymptomatic brain metastases. In the phase two trial, among ALK-positive treatment-naïve patients, 90% of patients achieved ORR (objective response rate), while 75% achieved IC-ORR (intracranial ORR). In the trial, among ALK-positive patients who previously underwent treatment with crizotinib with or without chemotherapy, 69% of patients achieved ORR, while 68% of patients achieved IC-ORR.
Among ALK-positive patients previously treated with a non-crizotinib ALK inhibitor with or without chemotherapy, 33% of patients achieved ORR, while 42% of patients achieved IC-ORR.
Among ALK-positive patients who previously received two or three ALK inhibitors with or without chemotherapy, 39% of patients achieved ORR, while 48% of patients achieved IC-ORR. Among ROS1-positive patients irrespective of previous treatment, 36% achieved ORR, while 56% achieved IC-ORR.
Some of the drugs that treat ALK-positive NSCLC include Novartis’s (NVS) Zykadia, Roche’s (RHHBY) Alecensa, Merck’s Keytruda, and Bristol-Myers Squibb’s (BMY) Opdivo. In 3Q17, Alecensa, Keytruda, and Opdivo generated revenues of $96 million, $1.0 billion, and $1.3 billion, respectively.
The Health Care Select Sector SPDR Fund (XLV) invests ~6.8%, 4.8% and 3.3% of its total portfolio holding in Pfizer, Merck, and Bristol-Myers Squibb, respectively.
