How Are Drugs Classified?
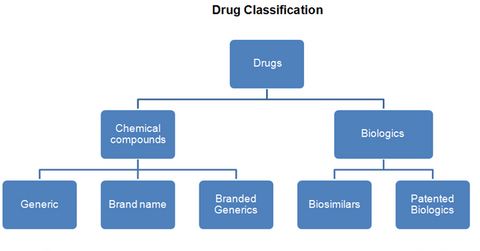
The two main classifications for drugs are chemical compound drugs and biologics. Medicinal drugs are broadly classified as prescription drugs and over-the-counter drugs.
July 2 2015, Published 11:14 a.m. ET
Drug classification
According to the Food, Drug, and Cosmetic Act of the U.S. Food and Drug Administration, a drug is “intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man or other animals.” Medicinal drugs are broadly classified into two categories: prescription drugs and over-the-counter (or OTC ) drugs. A pharmacist can’t dispense a prescription drug without a written prescription from a qualified healthcare professional.
The two main classifications for drugs are chemical compound drugs and biologics.
Chemical compound drugs
Chemical compound drugs are made by combining chemical ingredients in a fixed proportion. Most of the big pharmaceutical companies such as Pfizer (PFE), GlaxoSmithKline (GSK), and Johnson & Johnson (JNJ) develop these small-molecule drugs.
A brand name drug is protected by a patent and can be produced and marketed only by the company that holds the patent. Once the patent expires, competitors may enter the market with their own versions of the drug as generics or branded generics. A branded generic is a generic drug marketed under a brand name that’s different than the generic.
Biologics
According to the Biotechnology Industry Organization (or BIO), “A biologic is manufactured in a living system such as a microorganism, or plant or animal cells. Most biologics are very large, complex molecules or mixtures of molecules.” Produced on a mass scale, these drugs require the highest safety and manufacturing controls. Since the living systems used to produce these drugs are highly sensitive to minor changes in their environment, there’s a substantially higher risk of contamination and batch failures than chemical compound drugs.
Biosimilars are generic versions of biologic drugs. They can be introduced into the market (IBB) after the expiration of an innovator’s drug patent. Unlike chemical compounds, it’s difficult to establish if the composition and effectiveness of a biosimilar is equivalent to the original biologic drug. Thus, only one biosimilar has been approved to date in the United States. In March 2015, Novartis (NVS) received approval for Zarxio, a biosimilar of Amgen’s (AMGN) Neupogen, which is used to reduce the risk of infections in cancer patients.
Specialty drugs are a biotechnology product developed to treat complex diseases such as cancer. They’re typically very expensive and can require special treatment. Examples include Gilead Sciences’ (GILD) Hepatitis C drug Harvoni, Biogen’s (BIIB) hemophilia drug Alprolix, and Celgene’s (CELG) cancer drug Abraxane.
