Why the Generic Industry’s Classification Is Still Evolving
The generics industry primarily caters to several large diseases in primary care. Healthcare is organized into three categories—primary, secondary, and tertiary.
March 19 2015, Updated 1:06 p.m. ET
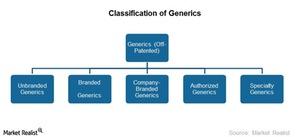
Classification of generics
The generics industry is the off-patented segment of the pharmaceutical industry. The industry is involved in developing, manufacturing, marketing, and distributing generic drugs. The generics market can be broadly categorized into four segments:
- Unbranded Generics
- Branded Generics
- Company-Branded Generics
- Authorized Generics
In recent years, a new segment emerged—Specialty Generics. We’ll discuss this segment in the next part of this series.
The generics industry primarily caters to several large diseases in primary care. Healthcare is organized into three categories—primary, secondary, and tertiary—depending on the nature of the health condition.
Primary care is focused on curing common illnesses—acute and chronic. Primary care is provided by physicians. Common chronic illnesses may include hypertension, diabetes, back pain, arthritis, and thyroid dysfunction. Secondary and tertiary are specialized care.
Please refer the above chart to compare unbranded, branded, and company-branded generics.
Unbranded generics
According to regulations, these are required to be bioequivalent. As a result, they don’t require a brand name. The substitution of unbranded generic medicines with other generics is determined by the pharmacist. In the US, Teva (TEVA) marketed ~375 generic products in 2013. It’s followed by Mylan (MYL). It has ~320 products. Other leading companies are Periggo (PRGO) and Impax (IPXL).
Branded generics
These are sold with a unique brand name other than the original proprietary trademark name. Branded generic drugs are only sold with a doctor’s prescription. As a result, a sales and marketing team is required to market the drugs to physicians.
Company-branded generics
These products are sold with a name that includes the name of the active ingredient and a company’s name as the prefix or suffix—either in whole or in part.
Authorized generics
These are generic versions of branded companies’ own patented drugs. They’re either marketed through its subsidiary or through another generic company. Actavis (ACT) is involved in the distribution of authorized generics.
The industry’s growth opportunities can be capitalized through pharmaceutical ETFs like the S&P Health Care Select Sector Index (XLV) and the SPDR S&P Pharmaceuticals ETF (XPH).
