Impax Laboratories Inc
Latest Impax Laboratories Inc News and Updates

Teva’s Recent Launch of Generic Solodyn: What You Need to Know
On February 20, 2018, Teva Pharmaceutical announced the US launch of the generic version of Solodyn Extended Release tablets in two strengths.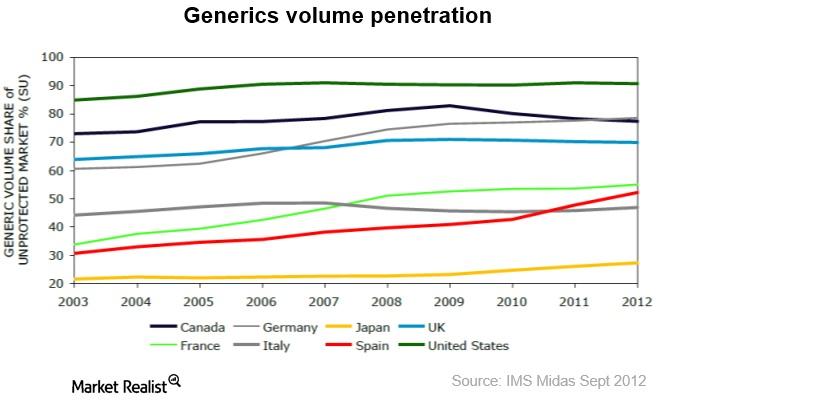
What’s Supporting Continued Growth in the Generics Market?
The global generics market was valued at $168 billion in 2013. From 2013 to 2018, it’s expected to grow at a CAGR of 11% to reach $283 billion.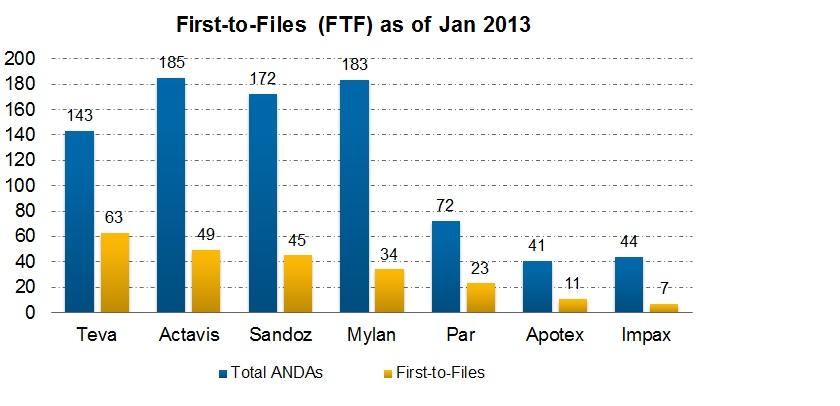
Is a Para IV Filing Rewarding for a Generic Company?
A generic company is rewarded for a Para IV filing. The first applicant to submit a substantially completed ANDA is given marketing exclusivity for 180 days.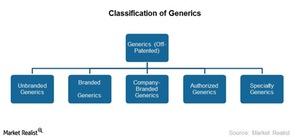
Why the Generic Industry’s Classification Is Still Evolving
The generics industry primarily caters to several large diseases in primary care. Healthcare is organized into three categories—primary, secondary, and tertiary.
Are Generics the Only Affordable Drugs?
Drugs are used to treat, cure, or prevent diseases. The drug market is broadly categorized into prescription drugs and OTC (over-the-counter) drugs.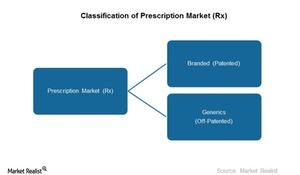
What Investors Need to Know about Branded and Generic Drugs
The prescription drug market is divided into two categories—branded or generic drugs. Branded drugs are patented drugs. Generics are off-patented drugs.
