Ignyta Inc
Latest Ignyta Inc News and Updates
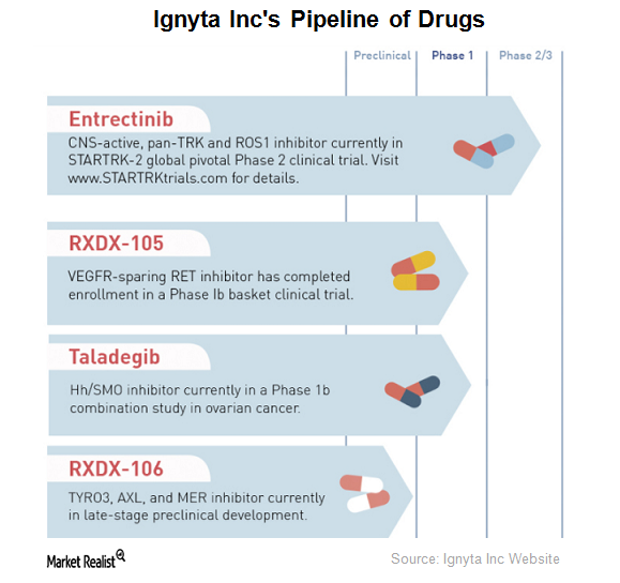
Ignyta’s Drug Pipeline
Ignyta (RXDX) has completed enrollment for a Phase 1 clinical trial of RXDX-105, an orally bioavailable small molecule tyrosine kinase inhibitor.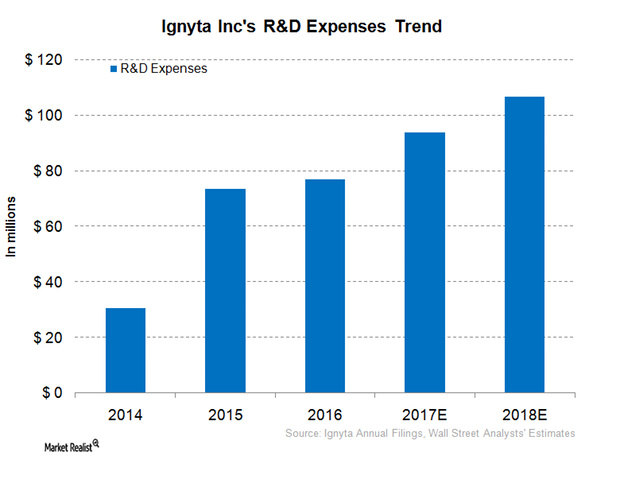
Inside Ignyta’s Financial Performance
Ignyta’s (RXDX) R&D (research and development) expenses increased from $16.6 million in 3Q16 to $21.7 million in 3Q17, a 30% rise.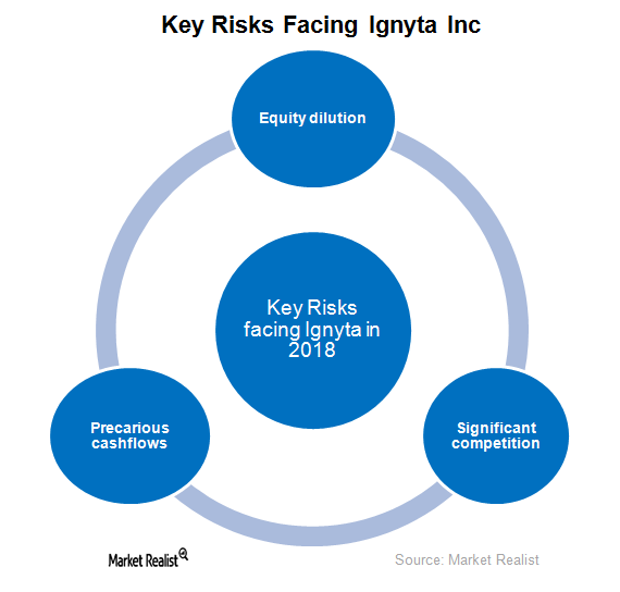
Ignyta and Its Key Risks in 2018
In October 2017, Ignyta (RXDX) raised $150 million by issuing 10 million shares of its common stock.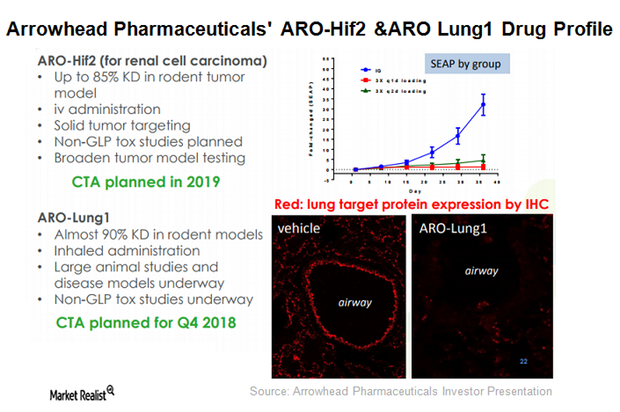
What Led to Arrowhead Pharmaceuticals’ Revenue Surge in 2017?
Arrowhead Pharmaceuticals’ therapeutic candidate ARO-LUNG1 is being developed for an undisclosed disease of the lung. This is the first candidate to utilize the company’s TRiM platform.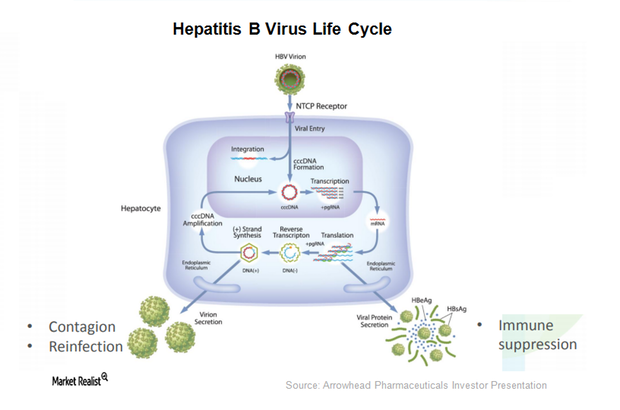
Arrowhead’s Candidates for Hepatitis B, Cardiovascular Diseases
ARO-HBV is Arrowhead Pharmaceuticals’ (ARWR) investigational drug candidate for treating chronic hepatitis B infection.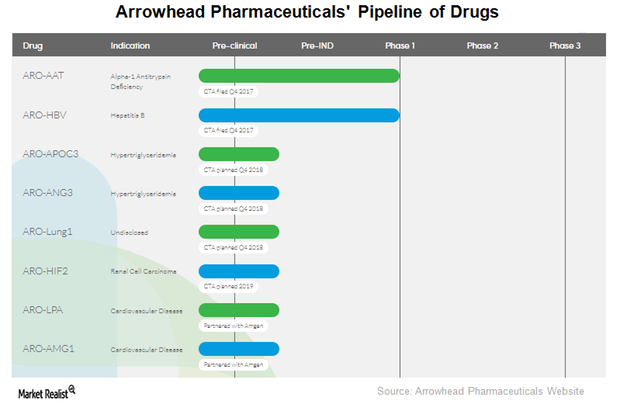
Taking a Closer Look at Arrowhead Pharmaceuticals’ TRIM Platform
Arrowhead Pharmaceuticals’ (ARWR) prior efforts were aimed at clinical programs that utilized the dynamic polyconjugate (or DPC), also called the EX1 delivery vehicle.
