Ajovy Is a New Growth Driver for Teva Pharmaceutical
On September 14, Teva Pharmaceutical (TEVA) issued a press release announcing FDA approval of humanized monoclonal antibody and anti-calcitonin gene-related peptide (or CGRP) therapy.
Dec. 4 2018, Updated 9:02 a.m. ET
Ajovy regulatory approvals
On September 14, Teva Pharmaceutical (TEVA) issued a press release announcing FDA approval of Ajovy, a humanized monoclonal antibody and anti-calcitonin gene-related peptide (or CGRP) therapy for migraine prevention indication. The drug has been approved in two dosages for monthly and quarterly administration. Ajovy was launched in the US market in the week starting September 24.
Teva Pharmaceutical expects to secure regulatory approval from the European Commission (or EC) for Ajovy in migraine prevention indication in H1 2019.
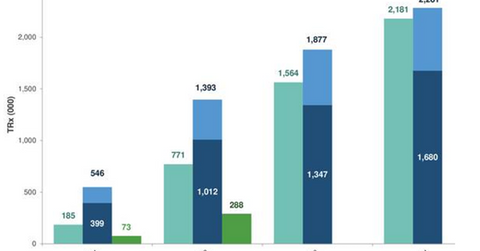
The above diagram highlights the strong Ajovy demand trends in the first few weeks after launch as compared to competing drugs such as Amgen (AMGN) and Novartis’s (NVS) Aimovig and Eli Lilly’s (LLY) Emgality.
According to Teva Pharmaceutical’s third-quarter earnings conference call, the normalized Ajovy prescriptions are adjusting for quarterly prescriptions of the drug. The company has witnessed 20% of Ajovy’s prescriptions written for quarterly administration.
Demand trends
At the end of the fourth week after its commercial launch, Ajovy’s total prescriptions (or TRx) had reached 4,439, while normalized TRx had reached 6,100. According to Teva Pharmaceutical’s third quarter earnings conference call, these did not include the samples that patients use to start therapy in the physician’s office.
According to Teva Pharmaceutical’s third-quarter earnings investor presentation, four weeks after launch, Ajovy was prescribed by around 1,600 healthcare practitioners. According to Teva Pharmaceutical’s third-quarter earnings conference call, 70% of the prescribers were either headache specialists or neurologists.
According to Teva Pharmaceutical’s third-quarter earnings investor presentation, the company’s patient support programs have also helped in providing easy access to the drug. According to Teva Pharmaceutical’s third-quarter earnings conference call, physicians have managed to secure samples of Ajovy withing one to two full days of ordering, and prescriptions are also being filled in a similar timeframe.
In the next article, we will discuss Austedo’s growth trends.
