Novartis’s Cosentyx May Emerge as a Leading Psoriasis Drug
According to Novartis’s estimates for the US biologics market in 2016, there were ~8.4 million patients with moderate to severe psoriasis in the US.
Feb. 16 2018, Updated 2:05 p.m. ET
Cosentyx in psoriasis indication
On January 16, 2018, Novartis (NVS) released data from its Phase 3b trial, CLARITY. This release highlighted the superiority of Cosentyx compared to Johnson & Johnson’s (JNJ) Stelara in clearing skin in patients with moderate to severe psoriasis at the end of 12 weeks and 16 weeks of therapy.
This trial is expected to be completed in 3Q18. Additionally, Novartis demonstrated the superior efficacy of Cosentyx compared to Stelara in clearing skin for moderate to severe psoriasis at the end of 52 weeks.
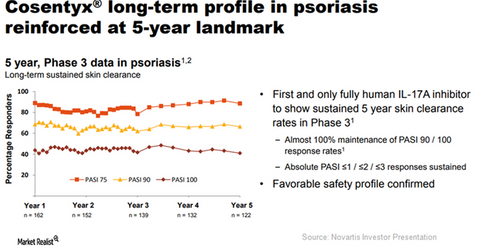
On September 13, 2017, Novartis released data from its Phase 3 trial. This data highlighted the ability of Cosentyx to maintain sustained skin clearance in moderate to severe psoriasis patients at the end of five years.
On March 6, 2017, Novartis announced data that showed that the majority of moderate to severe psoriasis patients who had paused Cosentyx treatment and had relapsed could regain response after 16 weeks of retreatment with Cosentyx.
This trend was due to almost zero immunogenicity or the absence of anti-secukinumab antibodies witnessed during the retreatment phase. Immunogenicity is a major challenge witnessed by many psoriasis biologic therapies.
Psoriasis market opportunity
According to Novartis’s estimates for the US biologics market in 2016, there were around 8.4 million patients with moderate to severe psoriasis in the US. Of these, around 1.7 million patients were diagnosed, which is ~20.0% of the total patient population.
Only 425,000 patients (25.0% of the diagnosed psoriasis patients) were treated with systemic therapy. Of these, around 164,000 (or 39% of the treated patients) were prescribed biologic therapies such as tumor necrosis factor (or TNF) inhibitors, anti-IL12-23 therapies, anti-IL17A therapies, and anti-IL23p19 therapies. AbbVie’s (ABBV) Humira and Amgen’s (AMGN) Enbrel are among the leading biologics indicated for psoriasis.
According to Novartis’s estimates about the EU5 biologics market in 2016, there were ~7.1 million patients with psoriasis in these countries. Of these, around 1.4 million patients were diagnosed, which is around 20.0% of the total patient population.
Only 300,000 patients, or 21% of the diagnosed psoriasis patients, were eligible for treatment with biologics. Of these, around 104,000, or 35% of the eligible patients, are prescribed biologic therapies. By creating awareness among dermatologists, Novartis plans to maintain leadership of Cosentyx in the US psoriasis market.
In the next article, we’ll discuss the growth opportunity for Cosentyx in the psoriatic arthritis indication.
