Pfizer’s Corporate and Pipeline Developments in 2Q17
Apart from Pfizer’s (PFE) product developments, let’s take a look at some recent pipeline and corporate developments.
By Mike Benson
Aug. 15 2017, Updated 10:36 a.m. ET
Other developments
Apart from Pfizer’s (PFE) product developments, let’s take a look at some recent pipeline and corporate developments.
Article continues below advertisement
Corporate developments
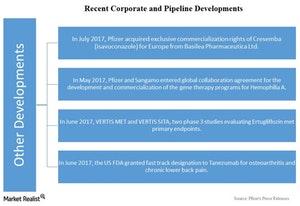
- In July 2017, Pfizer entered into a licensing agreement with Basilea Pharmaceutica for exclusive commercialization rights of Cresemba (isavuconazole) for Europe. Cresemba is an antifungal treatment for invasive aspergillosis and mucormycosis.
- In May 2017, Pfizer entered into a global collaboration and license agreement with Sangamo for the development and commercialization of the gene therapy programs for Hemophilia A.
Pipeline developments
- Pfizer and Merck (MRK) announced the two Phase 3 studies—Vertis Met and Vertis Sita, which were evaluating Ertugliflozin for improving glycemic control in patients with Type 2 diabetes—met their primary endpoints in June 2017.
- Pfizer announced that the Reflections B7391003 study—which evaluated PF-06439535, a proposed biosimilar bevacizumab, for safety and efficacy compared to Avastin (bevacizumab)—met its primary endpoints in July 2017.
- Pfizer and Eli Lilly and Co. (LLY) announced that Tanezumab received fast-track designation from the US Food and Drugs Administration (or FDA) for the treatment of osteoarthritis and chronic lower back pain in June 2017.
The iShares Core High Dividend ETF (HDV) invests 5.0% of its total assets in Pfizer (PFE). HDV also invests 3.1% of its portfolio in Merck and Co. (MRK) and 5.8% in Johnson & Johnson (JNJ).
