Farxiga Could See Robust Demand in International Markets in 2017
In 1Q17, AstraZeneca’s (AZN) Farxiga reported sales of $42 million in emerging markets, which equals year-over-year growth of ~100% on a reported basis.
July 11 2017, Updated 9:07 a.m. ET
Farxiga in emerging markets
In 1Q17, AstraZeneca’s (AZN) Farxiga reported sales of $42 million in emerging markets, which equals year-over-year (or YoY) growth of ~100% on a reported basis and 90% YoY growth on a constant currency basis. This is mainly attributed to an increasing number of commercial launches and reducing access constraints in these markets.
Farxiga (Dapagliflozin), marketed as Forxiga outside the US, secured regulatory approval in China in March 2017. The drug has become the first sodium-glucose co-transporter 2 (or SGLT2) therapy for type-2 diabetes in China.
In 1Q17, Farxiga reported revenues of ~$50 million in Europe, which represents YoY growth of ~22% on a reported basis and 24% on a constant currency basis. The drug also witnessed $7 million in sales in the Japanese market, where it is commercialized by AstraZeneca in partnership with Ono Pharmaceutical Co.
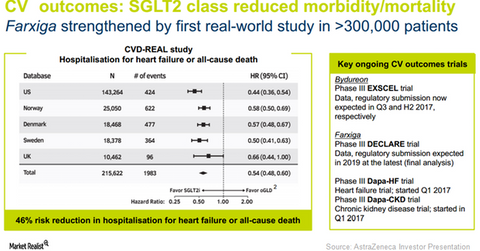
Encouraging cardiovascular outcomes data from the CVD-REAL study for the SGLT2 class of drugs in type-2 diabetes patients can play a pivotal role in boosting adoption of Farxiga in the US, as well as in international markets. This may have a favorable impact on AstraZeneca (AZN) stock as well as the VanEck Vectors Pharmaceutical ETF (PPH). AstraZeneca makes up about 4.7% of PPH’s total portfolio holdings.
Qtern approval
To compete effectively with type-2 diabetes players such as Johnson & Johnson (JNJ), Merck & Co. (MRK), and Eli Lilly & Co. (LLY), AstraZeneca has developed an oral tablet, Qtern, which is a fixed-dose combination of Farxiga and Onglyza.
On February 28, 2017, AstraZeneca announced that it had secured regulatory approval for Qtern from the FDA. Qtern would be used as an adjunct to diet and exercise to control blood sugar levels in type-2 diabetes patients who responded inadequately to Farxiga 10 mg monotherapy or those who had already been treated with Farxiga and Onglyza.
In the next article, we’ll discuss the growth prospects for AstraZeneca’s Bydureon.
