Upadacitinib: Opportunity for AbbVie’s Long-Term Growth?
ABBVie anticipates upadacitinib to be commercialized by 2019. If approved, AbbVie’s upadacitinib will directly compete with Pfizer’s (PFE) Xeljanz.
Aug. 7 2017, Updated 9:08 a.m. ET
About upadacitinib
AbbVie’s (ABBV) upadacitinib is a selective Janus kinase-1 inhibitor presently under investigation for the treatment of rheumatoid arthritis, ulcerative colitis, psoriatic arthritis, and Crohn’s disease.
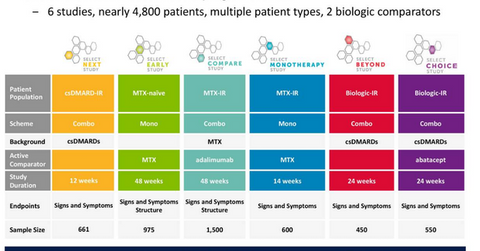
SELECT-NEXT trial
In July 2017, AbbVie presented the results of its phase 3 SELECT-NEXT clinical trial. AbbVie conducted the trial for the evaluation of the safety and efficacy of upadacitinib for the treatment of patients with moderate to severe rheumatoid arthritis who did not adequately respond to traditional synthetic DMARDs (disease modifying anti-rheumatic drugs).
At 12 weeks, 64.0% and 66.0% of the patients receiving upadacitinib 15 mg (milligrams) and upadacitinib 30 mg, respectively, achieved 20.0% improvement in the American College of Rheumatology Criteria (or ACR20) compared to 36.0% for patients under the placebo.
Among patients receiving upadacitinib 15 mg and upadacitinib 30 mg, 38.0% and 43.0% of patients, respectively, achieved ACR50 (50.0% improvement in the American College of Rheumatology criteria), while 21.0% and 27.0% of patients achieved ACR70 (70.0% improvement in the American College of Rheumatology criteria), respectively. Only 15.0% and 6.0% of the patients on a placebo achieved ACR50 and ACR70, respectively.
At week 12, 48.0% of the patients receiving either upadacitinib 15 mg or upadacitinib 30 mg achieved low disease activity (or LDA) compared to only 17.0% of the patients receiving a placebo. Further, 31.0% and 28.0% of the patients receiving upadacitinib 15 mg and upadacitinib 30 mg, respectively, achieved clinical remission compared to 10.0% of patients receiving a placebo. In the SELECT-NEXT study, both doses of upadacitinib met all primary and secondary clinical endpoints.
Ongoing trials with upadacitinib
AbbVie is conducting a phase 3 SELECT-MONOTHERAPY trial for the evaluation of upadacitinib as a monotherapy for the treatment of individuals with rheumatoid arthritis who did not have an adequate response to methotrexate.
AbbVie is also conducting another phase 3 SELECT-BEYOND trial to compare upadacitinib to a placebo in individuals with rheumatoid arthritis on a stable dose of standard synthetic DMARDs who did not adequately respond or were intolerant to biologic DMARDs.
ABBVie anticipates upadacitinib to be commercialized by 2019. If approved, upadacitinib will directly compete with Pfizer’s (PFE) Xeljanz. In the anti-rheumatoid arthritis drugs market, AbbVie’s upadacitinib could also face stiff competition from Johnson & Johnson’s (JNJ) Remicade, Pfizer’s Celebrex, Amgen’s (AMGN) Enbrel, Bristol-Myers Squibb’s Orencia, and others. The Vanguard Health Care ETF (VHT) invests ~3.13% of its total portfolio holdings in AbbVie.
