Edwards Lifesciences Focuses on Launch of SAPIEN 3 Ultra and CENTERA Valves
Edwards Lifesciences’ (EW) SAPIEN 3 Ultra system is a next-generation platform, with expandable Axela sheath technology and on-balloon delivery design.
Aug. 3 2017, Updated 9:06 a.m. ET
SAPIEN 3 Ultra system
Edwards Lifesciences’ (EW) SAPIEN 3 Ultra system is a next-generation platform, with expandable Axela sheath technology and on-balloon delivery design. This innovation is expected to improve both physician experience, as procedures will be faster and less prone to mistakes.
The new sheath technology is also expected to significantly reduce natural complications. Hence, the company believes that after its launch in late 2017, patients may migrate from SAPIEN 3 and other transcatheter heart valves (or THVs) to this new device for transcatheter aortic valve replacement (or TAVR) procedure.
Increasing awareness about the transcatheter aortic valve replacement (or TAVR) procedure is expected to reduce tolerance for symptoms in aortic stenosis (or AS) patients, who could not be treated by surgery until now. Being minimally invasive and also highly effective, asymptomatic and moderately severe AS patients may also start opting for TAVR therapy.
The changing dynamics in the AS space coupled with continuous innovations in the SAPIEN 3 heart valve may help boost Edwards Lifesciences’ stock price. It may also benefit the Vanguard Mid-Cap Growth ETF (VOT), as the company makes up about 1.5% of the ETF’s total portfolio holdings.
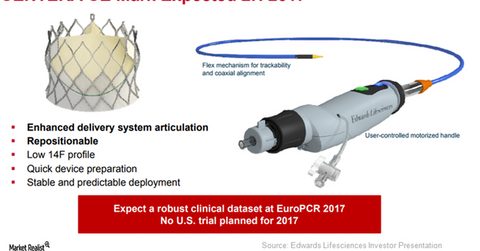
CENTERA system
On May 17, 2017, Edwards Lifesciences announced data that demonstrated the efficacy of its premium, self-expanding, CENTERA valve in 203 severe symptomatic AS (or ssAS) patients undergoing the TAVR procedure. The treated patients witnessed a high survival rate of ~around 99% and a low disabling stroke rate and permanent pacemaker rate of 2.5% and 4.9%, respectively.
Data from this trial also showed a very low 0.6% moderate paravalvular leak rate, while there were no reported cases of a severe paravalvular leak. Based on these promising results, Edwards Lifesciences plans to complete a limited launch of this device in Europe in late 2017. The company then plans to formulate its US launch strategy based on reception of the CENTERA system in Europe.
The SAPIEN 3 Ultra and CENTERA systems are expected to enable Edwards Lifesciences to compete more effectively with TAVR players such as Medtronic (MDT), Abbott Laboratories (ABT), and Boston Scientific (BSX).
In the final article in this series, we’ll discuss Edwards Lifesciences’ ongoing clinical trials in TAVR space in greater detail.
