How Cosentyx Resolves Enthesitis in Psoriatic Arthritis Patients
Only 525,000 patients (or 55% of the diagnosed psoriatic arthritis patients) were eligible for treatment with biologics.
Feb. 16 2018, Updated 3:35 p.m. ET
Cosentyx in psoriatic arthritis indication
On November 14, 2016, Novartis (NVS) announced positive results from the Phase 3 pivotal study, FUTURE 1. This study highlighted the efficacy of Cosentyx in providing sustained benefit over three years in psoriatic arthritis patients.
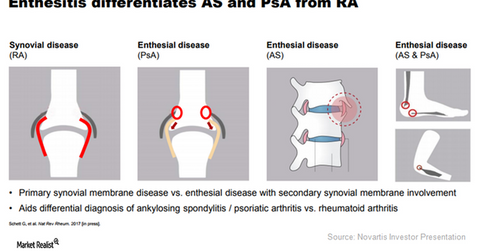
The drug works by inhibiting the IL-17A cytokine responsible for enthesitis in psoriatic arthritis patients. If left untreated, this can lead to irreversible structural damage. The chart below shows how enthesitis differentiates ankylosing spondylitis and psoriatic arthritis from rheumatoid arthritis.
Cosentyx is focused on not only resolving the signs and symptoms of this disease but also on constraining the structural progression of psoriatic arthritis condition. Unlike Cosentyx, traditional agents have generally worked to provide partial relief from symptoms such as pain and joint stiffness.
On November 7, 2017, Novartis announced positive data from its FUTURE 5 trial. This trial highlighted the potential of Cosentyx in inhibiting joint structural damage in psoriatic arthritis indication.
Novartis is also involved in recruiting patients into its head-to-head study, EXCEED, which compares the efficacy of Cosentyx with AbbVie’s (ABBV) Humira in psoriatic arthritis indication.
Psoriatic arthritis market opportunity
According to Novartis’s estimates about the US biologics market in 2016, there were ~1.6 million patients with psoriatic arthritis in the US. Of these, around 1.0 million patients were diagnosed, which is ~63.0% of the total patient population.
Only 535,000 patients, or 54% of the diagnosed psoriasis patients, were treated with systemic therapy. Of these, around 100,000 (or 19% of the treated patients) were prescribed biologic therapies such as tumor necrosis factor (or TNF) inhibitors, anti-IL12/23 therapies, and anti-IL17A therapies. Johnson & Johnson’s (JNJ) Simponi is another of the biologics approved in the psoriatic arthritis indication.
According to Novartis’s estimates about the EU5 biologics market in 2016, there were around 1.5 million patients with psoriatic arthritis in these countries. Of these, ~950,000 patients were diagnosed, which comprises ~63.0% of the total patient population.
Only 525,000 patients (or 55% of the diagnosed psoriatic arthritis patients) were eligible for treatment with biologics. Of these, around 97,000, or 18% of the eligible patients, are prescribed biologic therapies. The low penetration of biologics in the psoriatic arthritis segment in the US and the EU5 countries highlights the scope of growth opportunity available for Cosentyx in this indication.
In the next article, we’ll discuss the growth prospects for Cosentyx in ankylosing spondylitis indication.
