Why AbbVie’s Upadacitinib Keeps Posting Strong Data for Rheumatoid Arthritis
On December 20, 2017, AbbVie (ABBV) reported positive top-line results from its phase-3 trial Select-Monotherapy.
Jan. 8 2018, Updated 9:03 a.m. ET
Rheumatoid arthritis indication
On December 20, 2017, AbbVie (ABBV) reported positive top-line results from its phase-3 trial Select-Monotherapy, which evaluated Upadacitinib monotherapy in patients suffering from moderate to severe rheumatoid arthritis (or RA) and did not respond sufficiently to a previous methotrexate treatment.
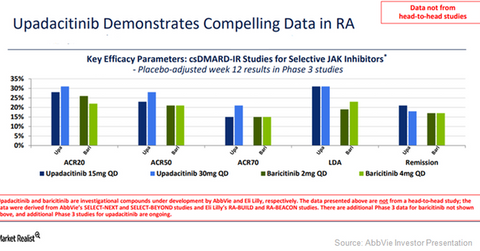
Methotrexate is generally used as a first-line treatment option for RA patients. After 14 weeks of treatment, both the 15 mg and 30 mg doses of Upadacitinib managed to reach a primary end point of an ACR 20 response and low disease activity (or LDA) as well as all ranked secondary end points in the trial.
On June 7, 2017, AbbVie reported positive results from the phase-3 trial Select-Next, which evaluated the clinical potential of investigational therapy, Upadacitinib, in patients suffering from moderate to severe RA and had not previously responded sufficiently to conventional synthetic DMARDs (or csDMARDs) therapy.
Upadacitinib managed to reach a primary end point of an ACR 20 response and low disease activity (or LDA) and all-ranked secondary end points in this trial.
Upadacitinib is expected to help AbbVie compete with other RA players like Pfizer (PFE), Amgen (AMGN), and Johnson & Johnson (JNJ).
Notably, AbbVie accounts for around 0.67% of the iShares Core S&P 500 ETF’s (IVV) total portfolio holdings.
Safety events
Besides AbbVie’s internal safety monitoring program, an independent data monitoring committee has been observing the Select study program to ensure patient safety. AbbVie also plans to generate a large body of safety data for Upadacitinib, since its study program will involve almost 3,000 patients receiving the therapy.
Despite increasing concerns about deep vein thrombosis (or DVT) and pulmonary embolism (or PE) event rates in the Select program (which is currently blinded data), AbbVie has been permitted to proceed with the program without modifications.
In the next part of this series, we’ll discuss the research program for Upadacitinib for atopic dermatitis.
