What Could Impact Oil Prices in 2018?
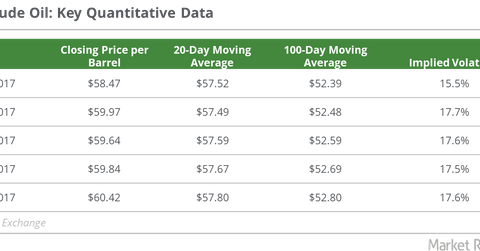
On December 29, 2017, US crude oil’s (USO) (USL) February 2018 futures rose 1% and closed at the 2017 highest closing price of $60.42 per barrel.
Nov. 20 2020, Updated 11:03 a.m. ET
US crude oil
On December 29, 2017, US crude oil’s (USO) (USL) February 2018 futures rose 1% and closed at their 2017 highest closing price of $60.42 per barrel. The fall in US crude oil inventories and US crude oil production could be behind oil’s gain.
In the week ended December 29, 2017, US crude oil inventories are expected to fall below 425 MMbbls (million barrels) for a further upside in oil prices. We’ll look at that more in Part 3 of this series. In the next part, we’ll analyze the influence of oil prices on US crude oil production.
A possible rise in US oil production may pose a threat to higher oil prices. Higher US crude oil exports could negatively impact oil prices globally. We’ll look at that in the last part of this series.
From December 22–29, 2017, US crude oil February futures rose 3.3%. Rising oil prices may influence the returns of the S&P 500 (SPY) and the Dow Jones Industrial Average (DIA).
Moving averages
On December 29, 2017, US crude oil active futures were 4.5%, 7.3%, 14.4%, and 19.9% above their 50-day, 20-day, 100-day, and 200-day moving averages, respectively. On the same day, US crude oil’s 50-day moving average was 11.7% higher than its 200-day moving average, which could indicate a further bullishness in oil prices.
