BRACAnalysis CDx Received FDA Approval for Ovarian Cancer Indication
On March 27, 2017, the FDA also approved BRACAnalysis CDX test as a complementary diagnostic test to be used with ovarian cancer maintenance therapy Tesaro’s (TSRO) Zejula (miraparib).
Dec. 4 2017, Updated 5:50 p.m. ET
Ovarian cancer indication
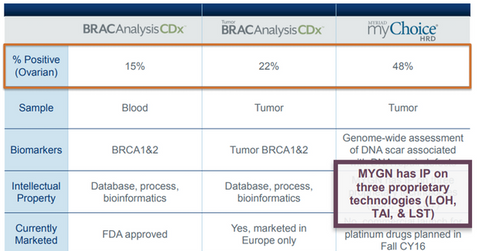
On December 19, 2014, Myriad Genetics’ (MYGN) BRACAnalysis CDx was approved by the U.S. Food and Drug Administration (or FDA) as a companion diagnostic test to identify deleterious or suspected deleterious germline BRCA mutations in ovarian cancer patients. This test helps recognize patients that stand to benefit from AstraZeneca’s (AZN) poly ADP ribose (or PARP) inhibitor therapy, Lynparza. On March 27, 2017, the FDA also approved BRACAnalysis CDX test as a complementary diagnostic test to be used with ovarian cancer maintenance therapy Tesaro’s (TSRO) Zejula (miraparib). Around 15% of the 20,000 epithelial ovarian cancer patients who are BRCA positive are expected to benefit from these approvals. Myriad Genetics makes up about 0.12% of the Vanguard Small-Cap Value ETF’s (VBR) total portfolio holdings.
On January 8, 2015, Myriad Genetics announced the launch of its Tumor BRACAnalysis CDx test on receipt of CE Mark in Europe. This was introduced to identify germline and somatic mutations in BRCA genes, thereby predicting the response to PARP inhibitors and platinum-based chemotherapy agents. This test could be used by close to 22% of ovarian cancer patients in Europe.
Finally, Myriad Genetics is also working on its myChoice HRD companion diagnostic test, which is a homologous recombination deficiency test. By measuring large-scale state transitions in cancer cells, telomeric allelic imbalance, and loss of heterozygosity parameters, the test aims to detect a tumor’s ability to repair double-stranded DNA breaks, which is a good indicator of a patient’s response to platinum-based chemotherapy or PARP inhibitors in multiple breast cancer and ovarian cancer indications. The company has projected the addressable market opportunity for this test to consist of 1.4 million patients across the US and Europe.
International market penetration
In addition to the premarket approval (or PMA) application in the US, Myriad Genetics has also submitted an application for regulatory and marketing approval of its BRACAnalysis CDx test in Japan as a companion diagnostic test with Lynparza in the metastatic HER2-breast cancer indication. If approved by the Pharmaceutical Medical Devices Agency and approved for marketing by the Ministry of Health, Labor and Welfare, it would add more than 10,000 eligible patients to Myriad Genetics’ addressable market on an annual basis. These approvals are also expected to boost the company’s financial guidance for 2018.
Peers such as Qiagen (QGEN) and Roche Holdings (RHHBY) have also focused on the Japanese market to expand the market reach of their companion diagnostic portfolio.
In the next article, we’ll discuss the riskScore offering in greater detail.
