Behind Exelixis’s Cabometyx Strategy for 2018
Exelixis (EXEL) expects the FDA’s approval for Cabometyx for first-line RCC (renal cell carcinoma) to be a major revenue driver.
Nov. 22 2017, Updated 7:33 a.m. ET
First-line renal cell carcinoma
Exelixis (EXEL) expects the FDA’s (US Food and Drug Administration) approval for Cabometyx for first-line RCC (renal cell carcinoma) to be a major revenue driver. Since the prescribers for first-line and second-line RCC therapy are the same in the US, the company expects to see a faster adoption of Cabometyx in new settings, driven by physicians who have previously prescribed the drug.
There are now ~14,000 patients eligible for first-line RCC therapy in the US, while there are ~10,500 and ~6,500 patients eligible for second-line and third-line RCC therapy, respectively. This implies that the first-line RCC therapy presents a growth opportunity for Cabometyx.
Notably, the treatment rates and duration of the therapy have been higher in the first-line RCC setting than in the second-line RCC treatment settings. This highlights the scope of market opportunity available for Cabometyx.
Exelixis accounts for 0.15% of the Vanguard Small-Cap ETF’s (VB) total portfolio holdings.
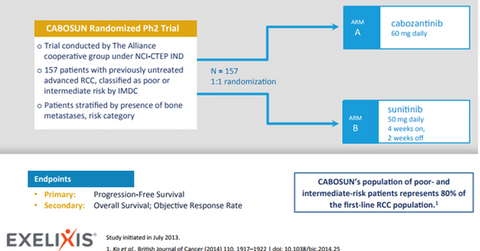
Cabosun trial
Cabometyx’s sNDA (supplemental new drug application) with the FDA for the first-line RCC indication is based on the randomized phase-2 Cabosun trial, which compared the efficacy of Cabometyx (cabozantinib) with Pfizer’s (PFE) Sutent (sunitinib) as a first-line RCC treatment in poor- and intermediate-risk patients. In addition to showing statistically significant improvements in investigator-assessed PFS (progression-free survival), radiographic images obtained by an independent radiology committee also confirmed the improvements in PFS with Cabometyx for this indication.
Exelixis is also exploring Cabometyx in combination with various immune checkpoint inhibitors offered by peers like Roche Holdings (RHHBY) and Bristol-Myers Squibb (BMY) in various cancer indications.
In the next part, we’ll discuss the growth prospects for Cabometyx for the hepatocellular carcinoma indication.
