Pfizer Sees a Significant Opportunity in This for Revenue Growth
In 2016, Pfizer’s (PFE) biosimilar business reported revenues of ~$319 million, compared with $63 million in 2015.
July 27 2017, Updated 7:36 a.m. ET
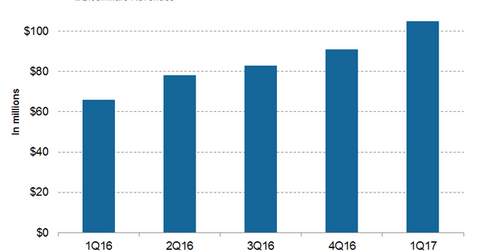
Biosimilar revenue trends
In 2016, Pfizer’s (PFE) biosimilar business reported revenues of ~$319 million, compared with $63 million in 2015. Pfizer’s Inflectra-Remsina generated revenues of ~$192 million in 2016, compared with $30 million in 2015.
In April 2016, the FDA (US Food and Drug Administration) approved Inflectra, a biosimilar of Johnson & Johnson’s (JNJ) Remicade. Inflectra is indicated for the treatment of individuals with moderate to severe Crohn’s disease who have had an inadequate response to traditional or standard therapy, ankylosing spondylitis, psoriatic arthritis, and chronic severe plaque psoriasis.
Inflectra is also used for the treatment of adult individuals with moderate to severe ulcerative colitis who had an inadequate response to standard traditional therapy. Inflectra in combination with methotrexate is indicated for the treatment of rheumatoid arthritis.
In 1Q17, Inflectra generated revenues of ~$78 million, compared with 36 million in 1Q16. In 1Q17, Pfizer’s biosimilars business generated net revenues of around $105 million, compared with $66 million in 1Q16.
Pfizer’s peers in the biosimilars market include Amgen (AMGN), Biogen (BIIB), Merck (MRK), and Teva Pharmaceuticals. The iShares Core High Dividend ETF (HDV) has ~5.0% of its total portfolio holdings in Pfizer.
Pfizer’s Nivestim and Retacrit
Pfizer’s other commercialized biosimilars include Nivestim and Retacrit. In 2016, Nivestim and Retacrit together generated revenues of ~$127 million, compared with $33 million in 2015. In 1Q17, Pfizer’s Nivestim and Retacrit together generated revenues of ~$27 million, compared with $30 million in 1Q16.
Pfizer commercializes Nivestim in certain Asian, European, and the Middle East and African markets. Nivestim (filgrastim) is a biosimilar of Amgen’s (AMGN) Neupogen. Nivestim is used reduce the duration of neutropenia in patients under established cytotoxic chemotherapy for malignancy. The drug is also used in patients undergoing myeloablative therapy after bone marrow transplantation.
Pfizer markets Retacrit in some European markets as well as in the Middle East and Africa. Retacrit is an epoetin zeta biosimilar indicated for the treatment of chemotherapy-induced symptomatic anemia.
