Where Abiomed Plans to Expand Impella CP’s Label
About 40% of patients succumb to heart failure within five years. Abiomed believes that it is reperfusion injuries that cause these problems.
July 25 2017, Updated 7:36 a.m. ET
STEMI patient segment
On October 27, 2016, the FDA (US Food and Drug Administration) approved the initiation of a feasibility study, STEMI DTU (door-to-unloading) study, aimed at studying the efficacy and safety of Impella CP heart pumps in unloading the left heart ventricle in patients before they undergo a PCI (percutaneous coronary intervention) procedure.
These patients are already suffering from STEMI (ST elevation myocardial infarction) but have not yet suffered from cardiogenic shock. On May 4, 2017, the company announced the enrollment of the first human subject in this trial.
This feasibility study is expected to form the basis for future pivotal trials, which will study the impact of unloading the left heart ventricle on infarct size which is associated with reperfusion, or tissue damage caused by a restored blood supply after ischemia or a period of oxygen deprivation.
Market opportunity
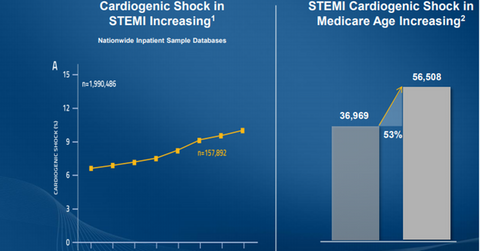
The above diagram shows the increasing incidence of STEMI cardiogenic shocks in all patients and in Medicare patients. This highlights the importance of Abiomed’s ongoing research efforts for this indication.
The company is targeting ~200,000 patients annually in the US, specifically those who are not currently being treated with Impella and have not witnessed cardiogenic shocks. Abiomed expects to complete an enrollment of 50 patients for its ongoing feasibility study across ten sites within the next 18 months.
If successful, the STEMI DTU feasibility study would result in a changed standard of care for these patients. Currently, hospitals deploy a door-to-balloon strategy wherein they open up the patient’s coronary artery within 90 minutes of a heart attack using an angioplasty balloon. While this technique has improved survival, it results in heart failure in 70% of these patients within five years of the first heart attack.
About 40% of patients succumb to heart failure within five years. Abiomed believes that it is reperfusion injuries that cause these problems, and continuous innovation would likely help Abiomed compete effectively with other cardiovascular players like Johnson & Johnson (JNJ), Medtronic (MDT), and Stryker (SYK).
Notably, the Vanguard Small-Cap ETF (VB) has about 0.18% of its total portfolio in Abiomed.
