How Are Eli Lilly’s New Products Performing?
Eli Lilly launched various products under different franchises. A few of the new products include Portrazza, Cyramza, Basaglar, Jardiance, and Taltz.
Sept. 5 2016, Updated 10:04 a.m. ET
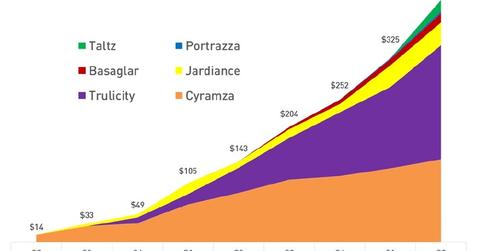
New products from Lilly
Eli Lilly and Co. (LLY) launched various products under different franchises. A few of the new products include Portrazza, Cyramza, Basaglar, Jardiance, Taltz, and Trulicity.
Basaglar
Basaglar is an insulin glargine injection to control blood sugar levels in patients with both type-1 and type-2 diabetes. Basaglar is expected to be launched in US markets in December 2016. Eli Lilly reported Basaglar sales of $16 million in 2Q16 in Japan and a few European markets.
Cyramza
Cyramza is an oncology drug used in combination with other drugs to treat metastatic NSCLC (non-small cell lung cancer), advanced gastric cancer, and metastatic colorectal cancer. Cyramza sales increased by more than 68% in 2Q16 to $147 million. Sales increased due to higher demand in Japan and European markets for the treatment of metastatic NSCLC and metastatic colorectal cancer.
Jardiance
Jardiance is another drug from Eli Lilly. It lowers blood sugar levels in patients with type-2 diabetes. Jardiance is part of Boehringer Ingelheim and Eli Lilly’s diabetes alliance. It reported revenues of $40 million for 2Q16—compared to $11 million for 2Q15.
Portrazza
Portrazza is a new drug to treat metastatic squamous NSCLC. The drug was launched in December 2015 after the FDA approved it on November 24, 2015. Portrazza reported sales of $4 million in 2Q16. To learn more, read FDA Approves Eli Lilly’s Portrazza based on Squire Study. The European Commission approved Portrazza in combination with other chemotherapy as a first-line treatment of non-small cell lung cancer on February 24, 2016. Recently, the drug launched in Europe.
Other drugs in the oncology field include Pfizer’s (PFE) Inlyta and Sutent, Bristol-Myers Squibb’s (BMY) Opdivo, Merck’s (MRK) Keytruda, GlaxoSmithKline’s (GSK) Mekinist and Tafinlar, and Roche’s Zelboraf.
Taltz
The company launched Taltz in US markets in April 2016. It launched Taltz in European markets in July 2016.
Trulicity
Trulicity is a drug that improves blood sugar levels in patients with type-2 diabetes. Trulicity reported sales of $201 million in 2Q16—compared to $44 million in 2Q15. The increase was mainly due to higher demand in US markets where the drug reported sales of $161 million during the quarter.
To divest the risk, investors can consider ETFs like the First Trust Capital Strength ETF (FTCS). FTCS holds ~2.1% of its assets in Eli Lilly.
