An Inside Look at Incyte’s Product Portfolio
Incyte’s (INCY) product portfolio includes drugs for oncology as well as non-oncology. For oncology, its product portfolio includes targeted therapies as well as immunotherapies.
June 10 2016, Updated 9:06 a.m. ET
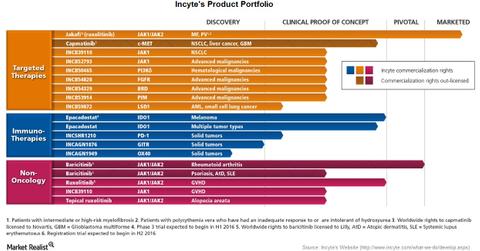
Incyte’s product portfolio
Incyte’s (INCY) product portfolio includes drugs for oncology as well as non-oncology. For oncology, its product portfolio includes targeted therapies as well as immunotherapies. However, the only product marketed by Incyte is Jakafi. Another product, baricitinib, is under pivotal studies for the treatment of rheumatoid arthritis.
The above chart shows the current status of Incyte’s drugs under development. As we’ve already seen, Jakafi is licensed to Novartis (NVS) for marketing outside the United States. Novartis markets Jakafi as Jakavi.
Worldwide rights for capmatinib are also licensed to Novartis. Worldwide rights for baricitinib are licensed to Eli Lilly (LLY).
Oncology products
Incyte has classified its oncology product portfolio into the following two categories. They’re based on the method of treatment used to cure a disease.
- targeted anti-cancer therapies
- immuno-oncology or immunotherapies
The targeted anti-cancer therapies include drugs that target cancer cells but cause less damage to normal cells. Jakafi and capmatinib are included in Incyte’s targeted therapy portfolio.
Immunotherapies target a patient’s immune system to fight cancer cells. Epacadostat is a drug in Incyte’s immunotherapy portfolio.
Non-oncology
Non-oncology drugs include baricitinib and ruxolitinib. Baricitinib is licensed to Eli Lilly.
In 1Q16, Eli Lilly filed a New Drug Application for baricitinib with the FDA (U.S. Food and Drug Administration). It also filed a Marketing Authorization Application with the EMA (European Medicines Agency).
In 1Q16, following these applications, Eli Lilly made a milestone payment to Incyte for baricitinib. Incyte will receive further milestone payments for the drug from Eli Lilly after the drug is approved. After that, Eli Lilly will pay royalties. AbbVie’s (ABBV) Humira is also used for the treatment of rheumatoid arthritis.
Incyte also announced an agreement with Eli Lilly for the development and commercialization of ruxolitinib for the treatment of graft-versus-host disease in US markets. It also entered into an agreement with Novartis for the development and commercialization rights of ruxolitinib outside the United States. The registration process for ruxolitinib in the United States will be initiated in the second half of 2016.
To reduce your risk, you can consider the PowerShares Dynamic Biotech & Genome ETF (PBE), which holds ~4.6% of its total assets in Incyte.
Next, let’s look at Incyte’s improved profitability and revised 2016 guidelines.
