BioMarin’s Pipeline for Duchenne Muscular Dystrophy
There are four drugs in the pipeline targeting Duchenne muscular dystrophy. The company holds Kyndrisa, BMN044, BMN045, and BMN053 for the indication of DMD.
Jan. 11 2016, Published 1:36 p.m. ET
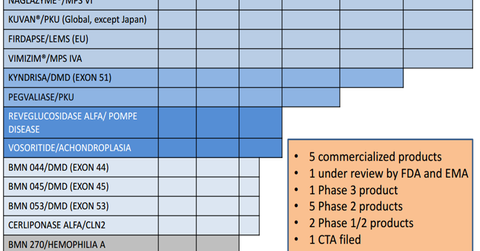
BioMarin’s research pipeline
During 2016, BioMarin anticipates launching three drugs. The three are Kyndrisa for Duchenne muscular dystrophy (or DMD), Pegvaliase for phenylketonuria (or PKU), and Cerliponase alfa for CLN2 disorder, a late infantile form of Batten disease.
The graph above details the pipeline of the company. Pegvaliase is a phase 3 drug. BioMarin (BMRN) plans to file a BLA (biologics license application) for Pegvaliase during the second half of 2016.
Acquiring Duchenne muscular dystrophy drugs from Prosensa
There are four drugs in the pipeline targeting Duchenne muscular dystrophy. The company holds Kyndrisa, BMN044, BMN045, and BMN053 for the indication of DMD.
These exon-skipping oligonucleotides were acquired from Prosensa. In 2009, Prosensa had entered into a collaboration agreement with GlaxoSmithKline P(GSK) for the development of its exon-skipping candidates. However, in 2014, Persona regained the rights to drisapersen and all other DMD candidates.
The graph above depicts the distribution of the DMD population that’s responsive to exon skipping. For further information on exon skipping, please see the article on how Kyndrisa would treat DMD.
As the graph suggests, ~80% of the population suffering from the disease has genotypes amenable to exon skipping.
Competition in DMD segment
In the DMD drug segment, major competitors of BioMarin include companies such as Sarepta Therapeutics (SRPT), PTC Therapeutics (PTCT), Nippon Shinyaku Co Ltd, a Japan-based company, as well as Santhera Pharmaceuticals Holding AG, a Switzerland-based company. Sarepta and BioMarin (BMRN) are testing the potential of exon 51 skipping to correct specific gene mutation. Thus, they both would target the same population subset. Approximately 13% of patients suffering from DMD might be responsive to exon 51 skipping.
Overview of Duchenne muscular dystrophy
Duchenne muscular dystrophy occurs exclusively in males. The disease results from the mutation in gene coding for dystrophin. Dystrophin is a protein required for proper muscle fiber function. Genetic testing helps to understand the exact nature and location of a dystrophin mutation.
The prevalence of the disease in the United States is ~18,000 wherea,s incidence for the disease is ~3,500 newborn boys. Boys suffering from the disorder show muscle weakness by the age of three years. This is followed by a progressive weakening of skeletal or voluntary muscles in the arms, legs, and trunk. Progressive respiratory failure is a major cause of death.
Kyndrisa, a drug that might become the first approved drug for patients who are responsive to Exon 51 Skipping, is the trade name for drisapersen. BioMarin has submitted an NDA (new drug application) to the FDA. The Prescription Drug User Fee Act (or PDUFA) date for the drug was fixed on December 27. However, the FDA notified the company that it would take action on NDA in early January 2016.
Investors can choose the NASDAQ-100 Equal Weighted Index Shares (QQQE) that holds 0.98% of its total holdings in BioMarin stock.
Investors can also choose the Vanguard Extended Market ETF (VXF). It holds 0.44% of its total holdings in BioMarin stock.
