Alexion Pharmaceuticals Diversifies Its Research Pipeline
Alexion Pharmaceuticals (ALXN) has strengthened its drug pipeline by diversifying its research programs across the metabolic disorder segment.
Sept. 29 2015, Updated 11:08 a.m. ET
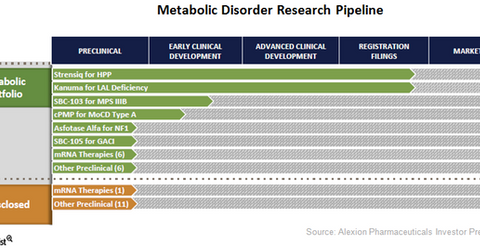
Diversified research pipeline
Alexion Pharmaceuticals (ALXN) has strengthened its drug pipeline by diversifying its research programs across the metabolic disorder segment. By 2018, the company plans to introduce up to eight new indications in the market, with the label expansion of Soliris combined with new discoveries in the metabolic segment.
The above diagram shows the various compounds and indications that Alexion Pharmaceuticals has explored in the metabolic segment and their respective development phases.
Strensiq and Kanuma
Strensiq and Kanuma were added to Alexion Pharmaceuticals’ portfolio in June 2015 in the acquisition of Synageva Pharmaceuticals. These drugs target metabolic diseases such as hypophosphatasia (or HPP) and Lysosomal Acid Lipase Deficiency (or LAL-D) and will enable Alexion to establish a premier rare metabolic disease franchise in the biotechnology industry. These two drugs are expected to be launched in 2015 and will lay the foundation of a multi-billion dollar franchise. Rare disease drugs such as Amgen’s (AMGN) Blinatumomab, Gilead Sciences’ (GILD) Zydelig, and Celgene’s (CELG) GED-0301 are also expected to be commercial successes for their respective companies, as they have more pricing flexibility than the mainstream drugs.
Other programs
Alexion has other enzyme replacement therapies such as SBC-103 and cPMP in the early stage pipeline of the metabolic segment. Alexion acquired the SBC-103 compound from Synageva Pharmaceuticals, which is being explored for Mucopolysaccharidosis type IIIB (or MPS IIIB). According to the Office of Rare Diseases Research, “Mucopolysaccharidosis type IIIB (MPS IIIB) is a genetic disorder that makes the body unable to break down large sugar molecules called glycosaminoglycans (GAGs, formerly called mucopolysaccharides).” Individuals with this disorder experience serious symptoms such as dementia, seizures, deafness, an inability to sleep for more than a few hours at a time, and several other neurological symptoms.
SBC-103 has been granted orphan drug designation (or ODD) by the Food and Drug Administration (or FDA) in April 2013 and the European Medicines Agency (or EMA) in June 2013. The therapy also received FDA’s Fast Track Designation in January 2015.
Other compounds such as cPMP and SBC-105 in early stage clinical development are being explored for rare metabolic diseases such as Molybdenum Cofactor Deficiency Disease Type A (or MoCD Type A) and generalized arterial calcification of infants (or GACI).
Investors can get exposure to Alexion Pharmaceuticals’ diversified research pipeline by investing in the iShares Nasdaq Biotechnology ETF (IBB). Alexion accounts for 5.07% of IBB’s total holdings.
