Drug Approval Process in the Biotechnology Industry
A drug approval process is required in order for a new drug to enter the market. Companies with new drugs in later stages of the approval process are more likely to earn a positive cash flow in the next few years.
July 7 2015, Updated 9:05 a.m. ET
Pipeline assets
Compared to the pharmaceutical industry, the biotechnology industry (IBB) is more research and development (or R&D) intensive. The fair value of a biotechnology company thus depends not only on its existing assets but also on its future growth assets.
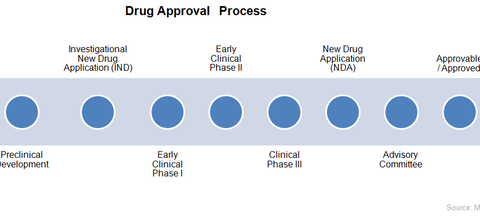
The above graph shows the FDA’s (Food and Drug Administration) drug approval process. The process is required in order for a new drug to enter the market. Companies with new drugs in later stages of the approval process are more likely to earn a positive cash flow in the next few years.
Preclinical studies
According to Tufts Center for the Study of Drug Development, it takes about 15 years for a new drug to go from the drug discovery phase to final distribution in the market. The process begins with drug discovery, an extremely capital-intensive, risky phase. It’s estimated that about one in 10,000 drug molecules in the drug discovery phase complete the approval process to enter the market.
In the preclinal development phase, the drug is studied in animals to ensure that it can be safely tested on humans. Toxicity, or the degree to which a drug can harm humans or animals, and metabolic pathways, or the sequence of chemical reactions that the drug undergoes in living organisms, is studied at this stage.
The overall probability that a drug in the discovery and preclinical phases will reach the final market is extremely low. So while calculating a company’s fair value, not much weight is given to drugs in the early phases of the approval process.
The biotechnology company then submits to the FDA an Investigational New Drug (or IND) Application with findings from the preclinal studies. Based on this information, the FDA either approves the clinical studies or asks the company to add more findings.
Clinical studies
Research has proved that out of 100 drugs that enter Phase I clinical studies, 70 will pass to Phase II, 33 will pass to Phase III, and about 25 will clear the last phase. In Phase I, the drug is tested for safety, dose ranges, and side effects on a small number of healthy males. The level of absorption of the drug in the body, called bioavailability, is also studied in this phase.
Phase II aims to evaluate the efficiency of the drug and the dosage required. It’s tested on a large number of the drug’s targeted audience. Phase III aims to confirm that the drug is effective and safe for the treatment of the targeted disease and that the dosage pattern is established.
Phase III aims to closely mirror the manner in which the drug will be used post marketing. This test is targeted toward a large number of patients and is costlier than the other phases. Many biologic companies such as Regeneron Pharmaceuticals (REGN), Amgen (AMGN), Biogen (BIIB), and Celgene (CELG) have various drugs, especially for cancer, rheumatoid arthritis, and Crohn’s disease, in the late phases of drug approval. These drugs are expected to earn substantial revenues in future years.
New drug application
After clinical trials, the company will then submit a New Drug Application (or NDA) to the FDA. Based on the research, the FDA decides whether to review the NDA in the next 60 days. The actual review will normally take 12 months, but the FDA can choose to review a breakthrough innovation within six months. Post approval, the FDA sends the NDA to an advisory committee consisting of experts capable of evaluating the drug.
Based on the advisory committee’s evaluation, the FDA announces whether the drug is approved or approvable. While approved drugs have no outstanding issues to be resolved, approvable drugs might need to resolve certain labeling or clinical study issues. In the case of approvable drugs, companies have to fulfill the requirements before the FDA will issue a formal approval for the drug.
