How Has Eli Lilly’s Cyramza Performed
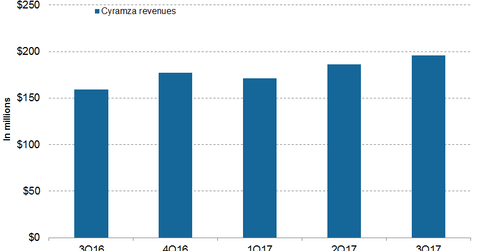
In 3Q17, Eli Lilly’s (LLY) Cyramza generated revenues of $196 million, which reflected ~23% growth on a YoY basis and 5% growth on a quarter-over-quarter basis.
Dec. 11 2017, Updated 7:31 a.m. ET
Cyramza revenue trends
In 3Q17, Eli Lilly’s (LLY) Cyramza generated revenues of $196 million, which reflected ~23% growth on a YoY basis and 5% growth on a quarter-over-quarter basis. In 3Q17, in the US and international markets, Cyramza reported revenues of $69.5 million and $126.5 million, respectively, compared to $67.0 million and $92.0 million in 3Q16.
Cyramza reported YTD September 2017 revenues of $553.5 million. In the US and international markets, Cyramza reported revenues of $204.3 million and $349.2 million, respectively.
REVEL trial
In October 2017, Eli Lilly presented new subgroup analysis of the phase 3 REVEL trial, which demonstrated the clinical benefits of Cyramza in the treatment of rapidly progressing advanced non-small cell lung cancer (or NSCLC).
In the REVEL trial, at nine weeks, 12 weeks, and 18 weeks, patients receiving Cyramza and docetaxel combination therapy demonstrated a median overall survival of 8.3 months, 9.1 months, and 8.5 months, respectively, compared to 4.8 months, 5.8 months, and 6.0 months for patients on placebo and docetaxel combination therapy.
In the trial, at nine weeks, 12 weeks, and 18 weeks, patients receiving Cyramza and docetaxel combination therapy demonstrated a median progression-free survival of 3.0 months, 3.6 months, and 3.2 months, respectively, compared to 1.5 months, 1.6 months, and 1.6 months for patients on placebo and docetaxel combination therapy.
Cyramza has U.S. Food and Drug Administration (or FDA) approval for the treatment of metastatic gastro-esophageal junction adenocarcinoma, metastatic non-small cell lung cancer, metastatic colorectal cancer.
Cyramza’s peers in the NSCLC drugs market include Roche’s (RHHBY) Avastin, AstraZeneca’s (AZN) Iressa, Bristol-Myers Squibb’s (BMY) Opdivo, and Boehringer Ingelheim’s Gilotrif. In 3Q17, Avastin, Iressa, and Opdivo reported revenues of $1.6 billion, $137 million, and $1.3 billion, respectively. The revenue growth of Eli Lilly’s Cyramza could boost the Vanguard High Dividend Yield ETF (VYM). Eli Lilly makes up about ~0.73% of VYM’s total portfolio holding.
