This Alone Could Boost Xyrem’s Addressable Market in 2018
To expand Xyrem’s addressable market, JAZZ has been evaluating the efficacy of Xyrem in pediatric narcolepsy patients with cataplexy and excessive sleepiness.
Aug. 22 2017, Updated 9:07 a.m. ET
Xyrem label revision
To expand Xyrem’s addressable market, Jazz Pharmaceuticals (JAZZ) has been evaluating the efficacy of Xyrem in pediatric narcolepsy patients with cataplexy and excessive sleepiness.
JAZZ presented positive data from its Phase-3 trial for Xyrem in this indication at the 31st Associated Professional Sleep Societies annual SLEEP meeting. The company plans to respond to the FDA’s (US Food and Drug Administration) written request to explore Xyrem in the pediatric population by filing an sNDA (supplemental new drug application) in 4Q17.
Phase-3 trial results
Jazz Pharmaceuticals has been evaluating Xyrem in pediatric narcolepsy patients with cataplexy in a Phase-3 randomized withdrawal trial. In this study design, pediatric narcolepsy patients naïve to Xyrem or already using this medication were either titrated up or stabilized and then subjected to two-week randomization period.
In the randomization period, these patients either continued with Xyrem or were administered a placebo. The trial also studied the extent of deterioration of the patients in the placebo arm compared with those who continued on Xyrem.
Primary endpoint
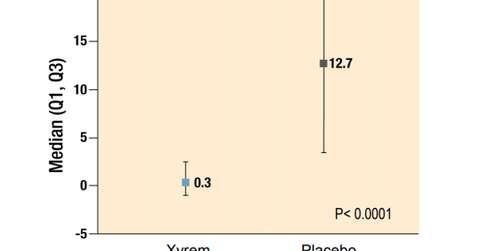
In the trial, the primary endpoint of weekly cataplexy attacks was seen to be in the range of 12–13 for the patients on placebo—significantly higher than those on Xyrem medication. The trial also showed that the patients in the placebo arm witnessed more sleepiness, as measured on the ESS (Epworth Sleepiness Scale) scale.
While Xyrem has demonstrated high efficacy in the pediatric population, its safety and tolerability profile was similar to that seen in the adult patient population.
Based on data from these trials, there’s a high probability of a label revision for Xyrem, which should strengthen JAZZ’s position against narcolepsy players like Teva Pharmaceuticals (TEVA), Mylan (MYL), and Allergan (AGN). Notably, the Vanguard Extended Market ETF (VXF) has ~0.24% of its total portfolio holdings in JAZZ.
In the next part of this series, we’ll discuss Jazz Pharmaceuticals other narcolepsy research programs.
