What Are the Medical Device Approval Processes in Major Markets?
In most countries, the approval process varies across different categories of devices classified as per their risk profiles.
Dec. 7 2015, Updated 5:04 a.m. ET
Medical device approval process
Medical devices are regulated by different countries as per their established regulatory processes and procedures to ensure device safety and efficacy. In most countries, the approval process varies across different categories of devices classified as per their risk profiles. Also, device approvals are required to be re-certified by most of the countries within regular intervals.
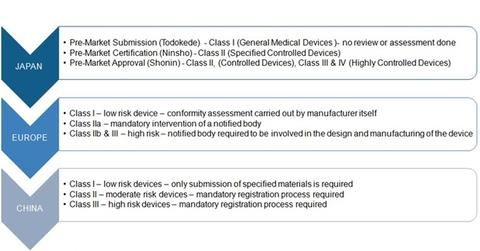
Japan
The Pharmaceutical and Medical Devices Agency (or PMDA) is the medical device regulatory body in Japan. Marketing business licenses called Marketing Authorization Holder (or MAH) licenses need to be obtained by companies intending to market medical devices in the Japanese market.
The Medical Device Registration system known as “Toroku” requires domestic companies to register their manufacturing facilities to the local agencies whereas foreign manufacturers need to register with PMDA.
Europe
In Europe, medical device companies need to conform to the requirements as specified under the Medical Devices Directive (or MDD). The European Union (or EU) doesn’t have a centralized regulatory body for device regulation and approval. EU members are responsible for ensuring that the medical devices being marketed in the EU region meet the required criteria.
Medical device companies classify the device as per its risk profile followed by an assessment of efficacy and safety by notified bodies. If the device is found to conform to specified standards, CE marking is provided.
China
In China, medical devices are regulated by the China Food and Drug Administration (or CFDA) and classified as per their risk levels. For Class I devices, submission of specified materials is the only requirement. Class II & III devices must follow a mandatory registration process, and the medical devices are required to undergo inspection and clinical tests, except those in the specified list of exempted devices.
Some of the US companies that market medical devices in foreign markets that earn significant revenues from exports are Medtronic (MDT), GE Healthcare (GE), Becton Dickinson (BDX), and Johnson & Johnson (JNJ). The iShares Dow Jones U.S. Medical Devices ETF (IHI) provides exposure to such companies in the US medical device industry.
