Patents and the Biotechnology Sector
The biotechnology industry will witness 12 blockbuster drugs worth $67 billion in annual revenues lose their patents by 2020. This is the so-called patent cliff.
July 7 2015, Updated 11:05 a.m. ET
Intellectual property rights
Intellectual property rights (or IPR) are related to an owner’s rights on creations of their intellect. These rights enable the owner to exclusively use these creations for a limited amount of time. Patents are a key mechanism of intellectual property protection in the biotechnology industry. The patent bestows on the owner monopoly rights related to new innovations, designs, and trade secrets for a given period of time.
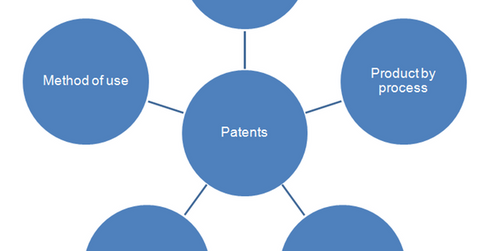
The above diagram shows the various types of drug patents. Product patent is the most lucrative option for the innovator biotechnology company. Patents are awarded for a period of 20 years, beginning on the date a drug owner applies for FDA approval of a new molecule. Since the approval process is lengthy, drugs generally enjoy monopoly rights for only 7–12 years.
Initially, a product patent is awarded for an innovative drug. This patent is related to composition of the drug and restricts other companies from copying the structure of the drug. Since the patent is in effect for a short period of time, a biotechnology company can attempt to extend the legal protection by applying for patents for other characteristics. This includes manufacturing of the drug by a particular process, innovative process, formulation, dosage pattern, method of use, or mode of administering the drug.
The need for patents
In 1980, The Supreme Court of the United States ruled that biological matter can be patented, thus allowing biotechnology companies to protect their inventions. Compared to other industries, the biotechnology industry (IBB) is an R&D- (research and development) intensive industry with a very low success rate. For every 5,000–10,000 drugs that enter the drug discovery phase, only one is expected to be approved and reach the market. With huge investment costs at stake, it becomes necessary for innovative biotechnology companies to protect their inventions from competitors.
The patent cliff
The biotechnology industry will witness 12 blockbuster drugs worth $67 billion in annual revenues lose their patents by 2020. This so-called patent cliff is when leading biotechnology companies face reduced sales when its established products lose patents and cheaper versions of the drug capture market share. With annual sales of $11 billion, AbbVie’s (ABBV) blockbuster drug Humira will witness patent expiration in 2016. Other major patent expirations through 2020 are Teva Pharmaceutical’s (TEVA) Copaxone, Amgen’s (AMGN) Neulasta, and Roche’s (RHHBY) Avastin.
