Probing Medtronic’s Research and Development Pipeline
Medtronic spent ~$1.6 billion—approximately 8.1% of its total sales—on research and development programs in fiscal 2015.
Dec. 4 2015, Updated 2:06 p.m. ET
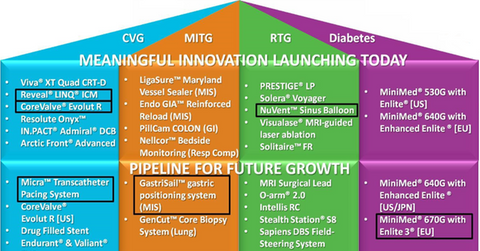
Medtronic’s research and development strategy
Innovation and differentiation are two of the most important factors determining the growth and success of any medical device company. With that in mind, Medtronic (MDT) spent ~$1.6 billion—approximately 8.1% of its total sales—on R&D (research and development) programs in fiscal 2015. Now, with the completed acquisition of Covidien in January 2015, Medtronic’s has one of the most highly diversified, largest product portfolios in the industry.
Medtronic’s recent product launches
The Reveal LINQ Insertable Cardiac Monitor is the smallest heart monitor on the market. The monitor automatically detects and monitors heart rhythms for up to three years, and it is anticipated that the device will contribute to significant revenue growth exceeding Medtronic’s expectations.
The CoreValve Evolut R is one of Medtronic’s other newly launched products. It is a self-expanding core valve that is able to be repositioned and is used for treating patients with aortic stenosis, a condition in which blood flow is restricted due to the narrowing of the aortic valve. The device has already demonstrated exceptional results over the past one year, yielding low rates of mortality and stroke.
Medtronic’s NuVent Sinus balloon, a system for sinus surgery, has a built-in electromagnetic surgical navigation technology. Although the company’s ENT (ear, nose, and throat) sales witnessed a slow growth in fiscal 2015, the NuVent Sinus balloon continued its strong penetration in the market.
Medtronic’s product pipeline
Medtronic’s Micra TPS (Transcatheter Pacing System) is the world’s smallest cardiac pacemaker, one-tenth the size of conventional pacemakers. During trials, no major complications were identified in 96% of the patients. The Micra TPS received a CE (European Conformity) marking in April 2015. In the US, clinical trial results have been sent to the FDA (Food and Drug Administration) for approval.
This product is expected to result in significant reductions in healthcare costs, although the Micra TPS faces stiff competition from the Nanostim leadless pacemaker from St. Jude Medical (STJ).
The GastriSail gastric positioning system is a surgical device that helps improve procedural efficiency during surgeries such as the sleeve gastrectomy, which is the most commonly performed weight loss surgery in the US. It was developed by Covidien, which was acquired by Medtronic in January 2015, and now forms part of the Minimally Invasive Therapies Group at Medtronic. The product is being launched in the US, Europe, and the Middle East and is expected to see an enormous demand in the market.
The MiniMed 670G
Medtronic’s MiniMed 670G with Enlite3 is a product that caters to type 1 diabetes patients, usually children. In type 1 diabetes, the body does not produce enough of the insulin hormone, which is necessary to carry glucose from the blood stream to the cells of the body. The device is undergoing trials and is expected to be launched in 2017 in the United States.
Competitors including Becton Dickinson and Company (BDX) and Abbott Laboratories (ABT) are also actively pursuing innovation through research and development and have a number of competing products in development or trial phase.
The Health Care Select Sector SPDR Fund (XLV) is a well-known ETF with an exposure of approximately 4% to Medtronic.
Continue to the next and final part of this series for a crucial glance at Medtronic’s marketing and sales strategy in 2015.
