Novo Nordisk’s Xultophy, Ryzodeg, and Fiasp Could Boost Revenue Growth in 2018
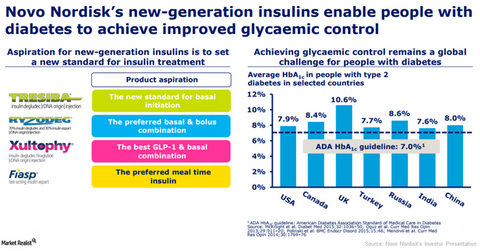
Novo Nordisk’s (NVO) Xultophy (insulin degludec and liraglutide combination) is used as an addition to diet and exercise for improvement of blood sugar levels in adults with type-2 diabetes mellitus whose blood sugar level could not be controlled adequately with basal insulin.
Jan. 4 2018, Updated 6:05 p.m. ET
About Xultophy
Novo Nordisk’s (NVO) Xultophy (insulin degludec and liraglutide combination) is used as an addition to diet and exercise for improvement of blood sugar levels in adults with type-2 diabetes mellitus whose blood sugar level could not be controlled adequately with basal insulin (less than 50 units daily). In December 2017, Novo Nordisk presented results of the quality-of-life questionnaires of the DUAL VII clinical trial. In the DUAL VII clinical trial, once-daily Xultophy demonstrated better diabetes management than multiple daily injections of basal insulin.
In the DUAL VII clinical trial, candidates on Xultophy demonstrated improved experiences for all diabetes-specific factors versus candidates on the basal-bolus therapy regimen. Patients on Xultophy demonstrated the highest improvement in treatment-related impact measure-diabetes (or TRIM-D) scores of 16.7 compared to 6.8 for patients on basal-bolus therapy. In the clinical trial, Xultophy had a treatment burden score of 12.4 compared to 4.3 for the basal-bolus therapy regimen. In the DUAL VII trial, the Short Form Health Survey 36 (or SF-36) questionnaire analysis found that Xultophy showed a statistically significant higher score compared to basal-bolus insulin regimen for the mental health component of the questionnaire, and all other comparisons were non-significant.
About Ryzodeg
Ryzodeg (insulin degludec and insulin aspart injection) is used for the improvement of blood sugar level in adults with diabetes mellitus. Ryzodeg is not indicated for diabetic ketoacidosis.
In December 2017, Novo Nordisk reported that Ryzodeg significantly decreased the risk of low blood sugar in individuals with type-2 diabetes who fast during Ramadan. Ryzodeg decreased the overall rate of low blood sugar including severe episodes by 62% and the rate of nocturnal hypoglycemia by 74% compared to biphasic insulin aspart 30 in individuals with type-2 diabetes who fast during Ramadan.
In September 2017, the US Food and Drug Administration (or FDA) approved Novo Nordisk’s Fiasp (fast acting insulin aspart) for the treatment of adults with diabetes. The U.S. FDA approval was based on the results from the clinical trials where Fiasp demonstrated an overall improvement in glucose management. Fiasp’s peers in the insulin market include Sanofi’s (SNY) Apidra and Eli Lilly’s (LLY) Humalog and Humulin.
The growth in sales of Novo Nordisk’s Xultophy, Ryzodeg, and Fiasp could boost the share prices of the Vanguard FTSE Developed Markets ETF (VEA). Novo Nordisk (NVO) and Novartis (NVS) both established players in the diabetes care market makes up ~0.42% and 0.89% of VEA’s total portfolio holdings, respectively.
