Why Jazz Pharmaceuticals’ Vyxeos Could Boost Revenue Growth in 2018
In August 2017, JAZZ’s Vyxeos liposome injection for the treatment of adult individuals with rapidly progressing or life t-AML received FDA approval.
Sept. 8 2017, Updated 7:37 a.m. ET
Approval of Vyxeos
In August 2017, Jazz Pharmaceuticals’ (JAZZ) Vyxeos liposome injection for the treatment of adult individuals with rapidly progressing or life t-AML (threatening acute myeloid leukemia) received FDA (US Food and Drug Administration) approval. The FDA has approved Vyxeos for a newly diagnosed therapy related to AML or AML with myelodysplasia-related changes, or AML-MRC.
About Vyxeos
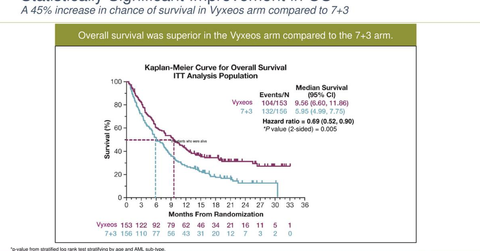
Vyxeos is a daunorubicin and cytarabine combination therapy. Its phase-3 pivotal trial evaluated the safety and efficacy of Vyxeos for the treatment of individuals with newly diagnosed t-AML, or AML-MRC, compared with cytarabine and daunorubicin (7+3). The 7+3 cytarabine and daunorubicin chemotherapy refers to cytarabine 100-200 mg/m2 on days one to seven and daunorubicin 60–90 mg/m2 on days one to three.
In the phase-3 pivotal trial, patients on the 7+3 (cytarabine and daunorubicin) treatment arm received induction with continuous infusion of cytarabine 100 mg/m2/day on days one to seven and daunorubicin 60 mg/m2/day on days one to three. Patients on Vyxeos received 44 mg/100 mg per m2 (daunorubicin and cytarabine) liposome intravenously through a 90-minute infusion on the first, third, and fifth day of induction and a 29 mg/65 mg per m2 (daunorubicin and cytarabine) liposome on days one and three.
In the phase-3 trial, patients in the Vyxeos therapy group and the 7+3 (daunorubicin and cytarabine) treatment group demonstrated median overall survival durations of 9.6 months and 5.9 months, respectively. Among patients on the Vyxeos therapy, 38% patients achieved complete response, compared with 26% patients on 7+3 (daunorubicin and cytarabine) therapy.
Jazz anticipates that Vyxeos will generate revenues of ~$10 million–$20 million. For more on Vyxeos’s sales prospects, please refer to Market Realist’s “How Jazz Pharmaceuticals Aims to Boost Vyxeos Sales in 2017.”
Jazz’s peers in the AML drug market include AbbVie (ABBV), Novartis (NVS), Celgene (CELG), Pfizer, and Agios Pharmaceuticals. Notably, the PowerShares Dynamic Pharmaceuticals Portfolio (PJP) has about ~2.4% of its total portfolio holdings in Jazz.
