What Amgen Expects from Blincyto in 2017
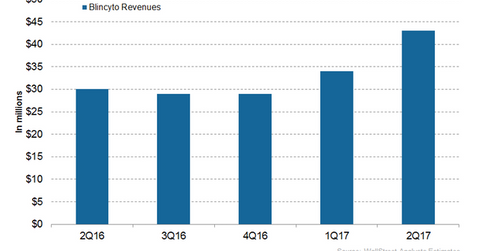
Blincyto’s revenue trends In 2Q17, Amgen’s (AMGN) Blincyto generated revenues of ~$43 million, which represented ~43% growth on a YoY (year-over-year) basis. Blincyto (blinatumomab) is an injection for the treatment of adults and children with relapsed or refractory B-cell precursor ALL (acute lymphoblastic leukemia). For more on Blincyto and its revenue prospects, please refer to […]
Aug. 16 2017, Updated 7:36 a.m. ET
Blincyto’s revenue trends
In 2Q17, Amgen’s (AMGN) Blincyto generated revenues of ~$43 million, which represented ~43% growth on a YoY (year-over-year) basis. Blincyto (blinatumomab) is an injection for the treatment of adults and children with relapsed or refractory B-cell precursor ALL (acute lymphoblastic leukemia).
For more on Blincyto and its revenue prospects, please refer to Market Realist’s “Blincyto Could Achieve High Volume Growth in 2017.”
Recent approval
In July 2017, the FDA (US Food and Drug Administration) approved Amgen’s sBLA (supplemental Biologics License Application) for Blincyto to include OS (overall survival) statistics from its phase-3 Tower trial. Amgen’s sBLA also included statistics from its phase-2 Alcantara trial, which showed the safety and efficacy of Blincyto in the treatment of individuals with Ph+ (Philadelphia chromosome-positive) relapsed or refractory B-cell precursor ALL.
This regulatory approval should help label expansion of Blincyto for the treatment of relapsed or refractory B-cell precursor ALL.
The Tower trial
The Tower trial demonstrated that patients on Blincyto showed a median OS of 7.7 months, compared with four months for patients on SOC (standard of care) chemotherapy. The Tower trial also demonstrated that 34% of the patients on Blincyto therapy achieved complete remission compared to 16% of patients on SOC chemotherapy.
Among the patients receiving Blincyto, 44% patients achieved CR (complete remission) or CR with partial or incomplete hematologic recovery, compared with 25% patients receiving SOC chemotherapy. Among patients with CR or CR with partial or incomplete hematologic recovery, 76% patients on Blincyto achieved MRD (minimal residual disease) negative status, compared with 48% of patients receiving SOC chemotherapy.
Notably, Amgen’s Blincyto faces competition from Novartis’ (NVS) Gleevec, Bristol-Myers Squibb’s (BMY) Sprycel, Ariad Pharmaceuticals’ Iclusig. In February 2017, Takeda Pharmaceuticals (TKPYY) completed the acquisition of Ariad Pharmaceuticals. Notably, the iShares Core S&P 500 ETF (IVV) invests ~0.60% of its total portfolio holdings in AMGN.
