SOLO 1 and SOLO 2 Trials Could Strengthen Dupixent’s Label
In Phase 3 trials, SOLO 1 and SOLO 2, Regeneron (REGN) and Sanofi (SNY) tested the efficacy of an investigational therapy, Dupixent, compared to a placebo.
Dec. 16 2016, Updated 9:06 a.m. ET
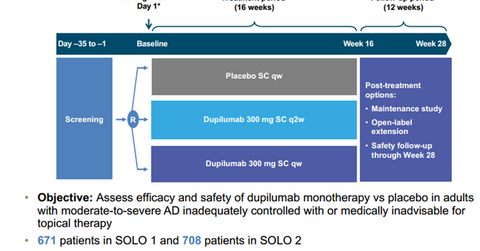
SOLO 1 and SOLO 2 trial design
In Phase 3 trials, SOLO 1 and SOLO 2, Regeneron (REGN) and Sanofi (SNY) tested the efficacy of an investigational therapy, Dupixent (Dupilumab), compared to a placebo. It was tested on patients suffering from moderate to severe AD (atopic dermatitis). The trials involved AD patients who failed to respond to or aren’t able to tolerate existing topical therapy options. Dupixent is expected to enable Regeneron to pose stronger competition to other biotechnology players such as Amgen (AMGN) and Biogen (BIIB).
The above diagram shows the design of the SOLO 1 and SOLO 2 trials—Dupixent 300 mg once a week and once every two weeks compared to a placebo.
Clinical trial results
In the SOLO 1 trial, 37% patients who were administered Dupixent once a week and 38% patients who were administered the drug once every two weeks managed to report clearing or near-clearing of skin lesions. In the SOLO 2 trial, 36% of the patients responded to both Dupixent once a week and once every two weeks. The use of a placebo once every week and once every two weeks resulted in clearing or near-clearing of skin lesions in 10% and 8% of the patients, respectively. Improvement in AD symptoms was measured with a five-point Investigator’s Global Assessment scale, which was also the co-primary endpoint for these trials.
The trial also posted positive results based on the second co-primary endpoint, which was a 75% improvement in the Eczema Area and Severity Index score—EASI-75. At the end of 16 weeks, 52% patients from the SOLO 1 study and 48% patients from SOLO 2 study on Dupixent once a week managed to achieve EASI-75 score. Also, 51% and 44% patients from the SOLO 1 and SOLO 2 studies, respectively, managed to achieve an EASI-75 score when administered with Dupixent once every two weeks. The performance was statistically significant compared to the placebo—15% and 12% of the patients managed to achieve an EASI-75 endpoint on the regimens of once a week and once every two weeks.
These trials might help secure approval for Dupixent from regulatory authorities in AD indication. It might have a positive impact on the company’s share prices as well as those of the iShares Core S&P 500 ETF (IVV). Regeneron accounts for about 0.15% of IVV’s total portfolio holdings.
In the next part, we’ll explore other research programs for Dupixent in more detail.
