Xalkori Label Expansion May Boost Pfizer’s Sales in 2016
On August 26, 2011, the FDA approved Pfizer’s (PFE) Xalkori for patients suffering from late-stage NSCLC and who express abnormal ALK gene.
Oct. 27 2016, Updated 10:05 a.m. ET
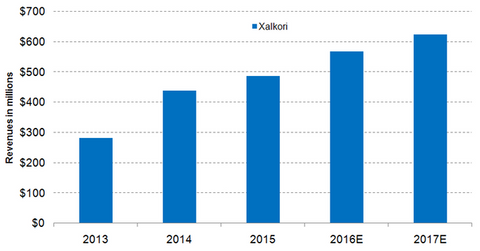
Xalkori’s label expansion
On August 26, 2011, the FDA (US Food and Drug Administration) approved Pfizer’s (PFE) Xalkori for patients suffering from late-stage NSCLC (non-small cell lung cancer) and who express abnormal ALK (anaplastic lymphoma kinase) gene. Xalkori’s label was further expanded on March 11, 2016, when the drug was approved by the FDA for metastatic NSCLC patients with ROS-1 gene alteration in their tumors.
Another FDA release claims that “ROS-1 gene alterations are present in approximately 1 percent of patients with NSCLC.” These statistics highlight the nature of commercial opportunity available for Xalkori in the NSCLC space.
Xalkori’s sales
Wall Street analysts estimate that Xalkori’s sales in 2016 will reach ~$567.7 million, which would be YoY (year-over-year) rise of about 16.3%. In 2015, Xalkori accounted for ~1.0% of Pfizer’s total revenues. Wall Street analysts expect that Xalkori will make up about 1.1% of Pfizer’s total revenues in 2016.
Xalkori’s revenues have been rising due to the increasing rate of diagnosis for ALK gene mutation in major markets. This trend is also expected to benefit other drugs targeting NSCLC with abnormal ALK gene expression such as Novartis’ (NVS) Zykadia and Roche Holdings (RHHBY) Alecensa (through its subsidiary Genentech).
Notably, Pfizer makes up about 1.1% of the total portfolio holdings of the iShares Core S&P 500 ETF (IVV).
New markets
On July 21, 2016, the Committee for Medicinal Products for Human Use also recommended the approval of Xalkori for metastatic NSCLC patients with ROS-1 gene alteration. After its approval by the European Medicines Agency, Xalkori will be launched in multiple European markets. Peers Eli Lilly (LLY) and AstraZeneca have also been actively involved in launching therapies in the NSCLC space.
In the next part, we’ll discuss the growth prospects of Inlyta in greater detail.
