Alnylam Pharmaceuticals: Developing Therapies for Hereditary ATTR Amyloidosis
Alnylam Pharmaceuticals (ALNY) is currently developing three investigational therapies—Patisiran, Revusiran, and ALN-TTRsc02—for the treatment of patients with hereditary ATTR amyloidosis.
Nov. 20 2020, Updated 3:47 p.m. ET
Hereditary ATTR amyloidosis
Alnylam Pharmaceuticals (ALNY) is currently developing three investigational therapies—Patisiran, Revusiran, and ALN-TTRsc02—for the treatment of patients with hereditary ATTR amyloidosis.
According to Ionis Pharmaceuticals’s (IONS) website, “Amyloidosis is a rare disease caused by the build-up of abnormal material called amyloid within the tissues of the body. Amyloid deposits cause illness by damaging the structure and the function of the organs where they are found and they can affect almost any part of the body.”
With a robust research pipeline, Alnylam Pharmaceuticals could pose strong competition to other rare disease players such as United Therapeutics (UTHR) and Vertex Pharmaceuticals (VRTX).
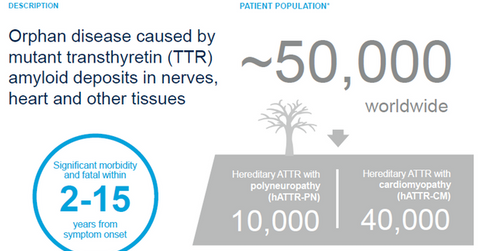
Market opportunity
Affecting around 50,000 people around the world, hereditary ATTR amyloidosis is further categorized in two subtypes: polyneuropathy and cardiomyopathy. Some patients display symptoms of both subtypes of the disease. Alnylam Pharmaceuticals recognizes mutant transthyretin (or TTR) protein as the root cause of hereditary ATTR amyloidoisis.
Patisiran is being developed for hereditary ATTR amyloidosis with polyneuropathy (or hATTR-PN). The company expects to witness results from its Phase 3 clinical trial, Apollo, testing Patisiran for hATTR-PN by mid-2017. If these results are positive, Alnylam Pharmaceuticals will submit the new drug application (or NDA) for Patisiran to the FDA in late 2017.
According to Alnylam Pharmaceuticals, “Specifically, hereditary ATTR amyloidosis with polyneuropathy (hATTR-PN), also known as familial amyloidotic polyneuropathy (FAP), is an inherited, progressive disease leading to death within 5 to 15 years.
“It is due to a mutation in the transthyretin (TTR) gene, which causes misfolded TTR proteins to accumulate as amyloid fibrils predominantly in peripheral nerves and other organs. hATTR-PN can cause sensory, motor, and autonomic dysfunction, resulting in significant disability and death.”
Research initiatives
Alnylam Pharmaceuticals is also conducting research on Revusiran for hereditary ATTR amyloidosis with cardiomyopathy (or hATTR-CM). According to Alnylam Pharmaceuticals, “Specifically, hereditary ATTR amyloidosis with cardiomyopathy (hATTR-CM), also known as familial amyloidotic cardiomyopathy (FAC), is an inherited, progressive disease leading to death within 2 to 5 years.
“It is due to a mutation in the transthyretin (TTR) gene, which causes misfolded TTR proteins to accumulate as amyloid fibrils primarily in the heart. Hereditary ATTR amyloidosis with cardiomyopathy can result in heart failure and death.”
The company is also developing a therapy that would be superior to both Patisiran and Revusiran, which is ALN-TTRsc02. If the drug manages to be successful in its clinical trials, it is expected to prove beneficial for the share prices of Alnylam Pharmaceuticals as well as the iShares Russell 1000 ETF (IWB). Alnylam Pharmaceuticals makes up about 0.02% of IWB’s total portfolio holdings.
In the next article, we will analyze the progress of Anylam Pharmaceuticals in the development of Patisiran for the treatment of hereditary ATTR amyloidosis with polyneuropathy.
