What Can You Expect from Markets in 2016?
While I don’t have a crystal ball, here are three things I believe all investors need to know about returns in 2016.
Feb. 17 2016, Updated 8:08 a.m. ET
What can we expect from markets this year?
With 2016 off to a rocky start so far, you may be wondering whether we’ll see more of the same this year. While I don’t have a crystal ball, here are three things I believe all investors need to know about returns in 2016.
1. Solid returns will remain hard to come by.
Unfortunately, as this year kicks off, many of the challenges that made positive return generation difficult in 2015 are likely to persist. In developed markets, these challenges include long-term interest rates that are still near multi-decade lows, as well as elevated equity valuations.
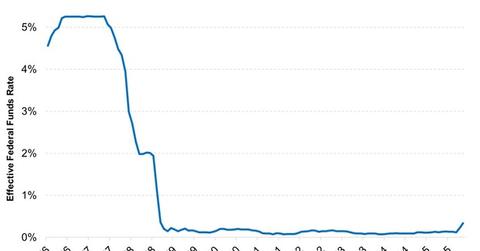
Indeed, even as the Federal Reserve (the Fed) began the process of rate normalization late last year, it left interest rates unchanged at its policy meeting this month. In fact, given that the U.S. labor market likely experienced its cyclical peak at the end of 2015 and the Fed began raising rates too late in my opinion, current Fed Funds futures are pricing in essentially only one hike in 2016, according to data accessible via Bloomberg. In short, rates will remain low for the foreseeable future.
Market Realist – Interest rates are likely to remain low as the global economy slows.
The Fed hiked the funds rate for the first time in ten years in December. We aren’t likely to see another hike soon.
While lower oil prices should be a net positive for the United States, the fact the oil prices are at a 12-year low has investors wondering and worrying about the state of the global economy. That being said, low oil prices are a result of the supply glut more than anything else.
Lower oil and gas prices have led to 67 oil and gas bankruptcies since 2015, according to Galvin/Solmonese, a consulting firm. These bankruptcies led to layoffs in the sector, which could have a negative effect on consumption. Also, banks (KRE)(IYF) with exposure to the energy sector (IYE) could see rising defaults. However, most major banks have no more than 6% debt exposure to the sector.
Meanwhile, another worry is the slowing growth in China (MCHI) and a possible contagion that could slow down global growth further.
All these factors, along with the markets’ volatile nature, mean that the Fed might think twice before hiking rates again. That’s why interest rates are likely to stay low.
As we mentioned in Part 1 of this series, the S&P 500 Index (IVV) has fallen over 10% already this year. Returns will be difficult to come by, with the stronger dollar (UUP) likely to keep exports muted. While the correction has made US equities (QQQ)(DIA) cheaper, there’s no visible catalyst to take markets higher from current levels. However, we’re also unlikely to see a scenario like 2008.
