Sage Therapeutics’ GABA Receptor–Based Products
On June 12, Sage Therapeutics announced its expedited development plan for SAGE-217.
July 13 2018, Updated 10:32 a.m. ET
Sage Therapeutics’ products
As we discussed earlier, Sage Therapeutics’ (SAGE) portfolio includes products under development for the treatment of life-threatening central nervous system (or CNS) disorders, based on two different programs: GABA receptor systems and NMDA receptor systems. The chart below shows the product portfolio for the GABA Receptor program.
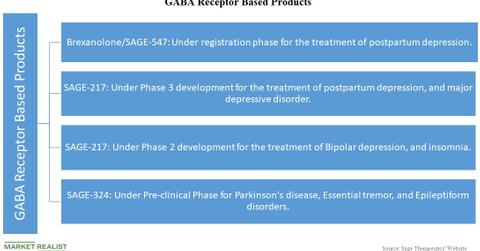
GABA Receptor–based products
Gamma-AminoButyric Acid (or GABA) is an inhibiting neurotransmitter in the CNS. The products using GABAA Receptor program, their uses, and status of development follow.
Brexanolone
Brexanolone (or SAGE-547) is the lead product candidate from Sage Therapeutics and is under the registration phase for the treatment of postpartum depression. On May 30, the FDA accepted the New Drug Application for Brexanolone IV, an allosteric modulator for synaptic as well as extra-synaptic GABAA receptors.
Brexanolone IV
Brexanolone IV also received Priority Review status from the FDA for the treatment of postpartum depression. The Prescription Drug User Fee Act (or PDUFA) target date is December 19. If approved, the drug would be the only approved medicine for the treatment of postpartum depression.
SAGE-217
SAGE-217 is a drug under Phase 3 development for the treatment of postpartum depression and major depressive disorder. It’s also under Phase 2 development for the treatment of bipolar depression and insomnia.
On June 13, Sage Therapeutics and Shionogi & Co. announced a strategic collaboration for the development and commercialization of SAGE-217. On June 12, Sage Therapeutics announced its expedited development plan for SAGE-217.
SAGE-324
SAGE-324 is a drug under development in pre-clinical stage for the treatment of Parkinson’s disease, essential tremor, and epileptiform disorders.
The SPDR S&P Biotech ETF (XBI) holds 1.3% of its total investments in Sage Therapeutics (SAGE), 1.3% in Gilead Sciences (GILD), 1.3% in BioMarin (BMRN), and 1.2% in Ionis Pharmaceuticals (IONS).
