Dupixent Could Substantially Drive Regeneron’s Growth
In March 2017, the FDA approved Regeneron and Sanofi’s Dupixent injection for the treatment of adult individuals with moderate to severe atopic dermatitis.
Aug. 1 2017, Updated 9:09 a.m. ET
Dupixent launched in the United States
In March 2017, the FDA approved Regeneron Pharmaceuticals’ (REGN) and Sanofi’s (SNY) Dupixent injection for the treatment of adult individuals with moderate to severe atopic dermatitis (or AD) whose disease conditions couldn’t be controlled via topical prescription therapies or whose conditions had treatments that weren’t advisable.
In 2014, the FDA designated Dupixent as a breakthrough therapy for the treatment of moderate to severe AD. In the United States, Dupixent’s wholesale acquisition cost (or WAC) is ~$37,000 annually.
The FDA approved Dupixent based on the global LIBERTY AD clinical program. The LIBERTY AD clinical program included three Phase 3 pivotal trials, SOLO 1, SOLO 2, and CHRONOS, which were conducted to evaluate the safety and efficacy of Dupixent for the treatment of moderate to severe AD.
SOLO 1 and SOLO 2 studies
In the SOLO 1 and SOLO 2 studies, Dupixent was evaluated as a monotherapy for the treatment of AD. At 16 weeks, in the SOLO 1 and SOLO 2 Phase 3 trials, 38% and 36% of patients who received Dupixent 300mg, respectively, achieved clear skin or almost clear skin measured by the 5-point Investigator’s Global Assessment (or IGA) scale, the primary endpoint of the study, compared to 10% and 9%, respectively, for patients on the placebo therapy.
At 16 weeks into the SOLO 1 and SOLO 2 trials, 51% and 44% of patients who received Dupixent 300mg, respectively, achieved a reduction of 75% or more in their Eczema Area and Severity Index scores (EASI-75), compared to 15% and 12% of patients on the placebo.
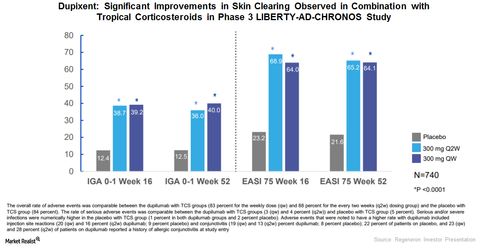
CHRONOS study
Regeneron Pharmaceuticals and Sanofi conducted the Phase 3 CHRONOS trial to evaluate the safety and efficacy of Dupixent in combination with topical corticosteroids (or TCS) compared to placebo and TCS combination for the treatment of severe to moderate AD.
At 16 weeks, among those patients who received Dupixent 300mg every two weeks, 39% achieved clear skin or almost clear skin, compared to 12% who received the placebo and TCS combination therapy.
In the CHRONOS trial, at 16 weeks, 69% of the patients who received the Dupixent 300mg and TCS combination therapy achieved EASI-75, compared to 23% of patients who received the placebo and TCS combination therapy.
Regeneron Pharmaceuticals’ peers in the dermatology drug market include Anacor Pharmaceuticals (ANAC), Bayer (BAYZF), AbbVie, and Mylan. The PowerShares Dynamic Pharmaceuticals ETF (PJP) has ~2.8% of its total portfolio holdings in Regeneron Pharmaceuticals.
