Myriad Genetics Inc
Latest Myriad Genetics Inc News and Updates
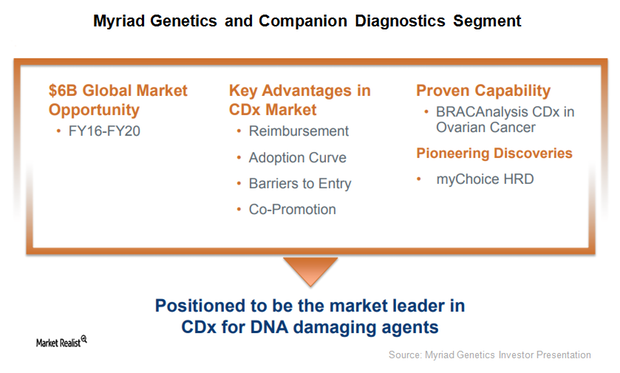
This Could Be a Solid Growth Driver for Myriad Genetics in 2018
Myriad Genetics (MYGN) announced the U.S. Food and Drug Administration’s (or FDA) acceptance of its supplementary premarket approval application for BRACAnalysis CDx, a DNA sequencing companion diagnostic test.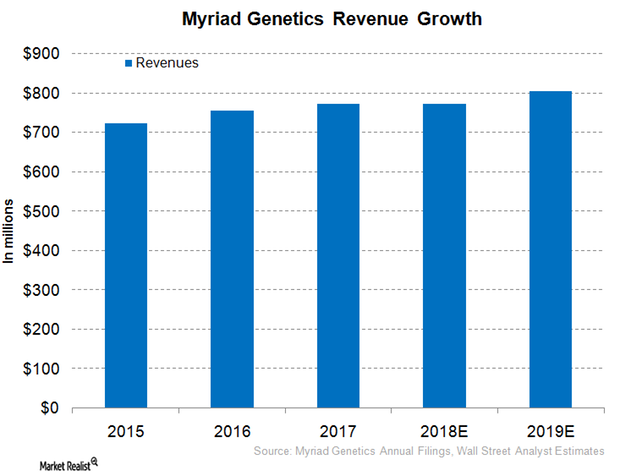
Myriad Genetics Expected to Report Flat Revenue Growth in Fiscal 2018
Myriad Genetics (MYGN) expects to report revenues in the range of $750 million–$770 million in fiscal 2018 (ended June 30, 2018).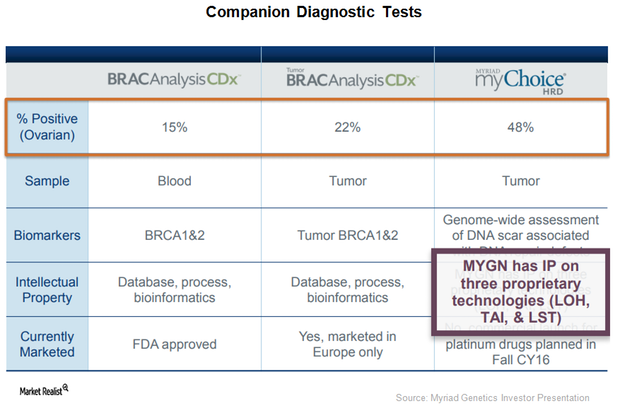
BRACAnalysis CDx Received FDA Approval for Ovarian Cancer Indication
On March 27, 2017, the FDA also approved BRACAnalysis CDX test as a complementary diagnostic test to be used with ovarian cancer maintenance therapy Tesaro’s (TSRO) Zejula (miraparib).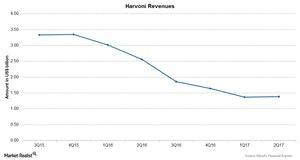
How Did Gilead’s Blockbuster Drug Harvoni Perform in 2Q17?
Harvoni is the top-selling drug in Gilead Sciences’ (GILD) portfolio. The drug is used for the treatment of genotype-1 hepatitis C virus (or HCV) infection.
