Momenta Pharmaceuticals Inc
Latest Momenta Pharmaceuticals Inc News and Updates
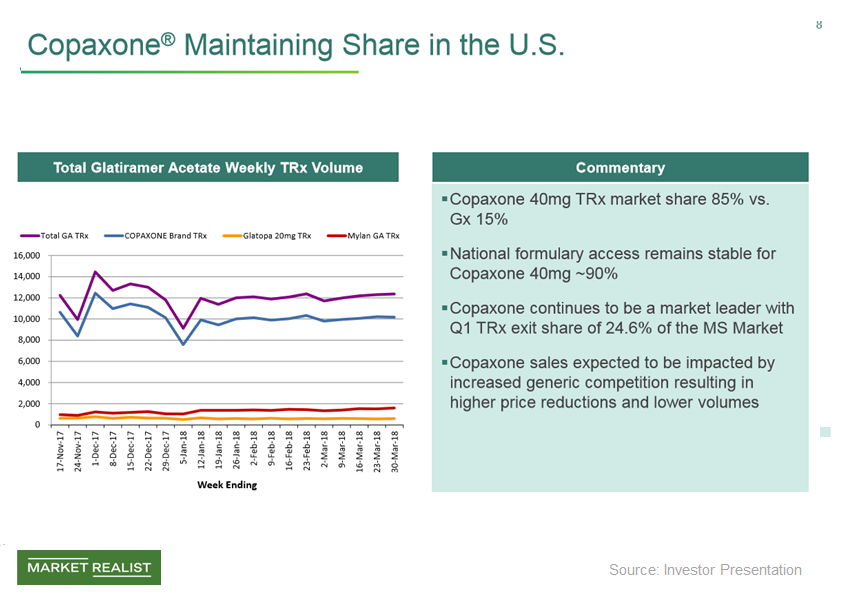
Teva’s Copaxone Maintains Market Share amid Intense Competition
In fiscal 1Q18, Teva (TEVA) reported sales of $645 million for its multiple sclerosis drug, Copaxone, a sequential decline of ~21%.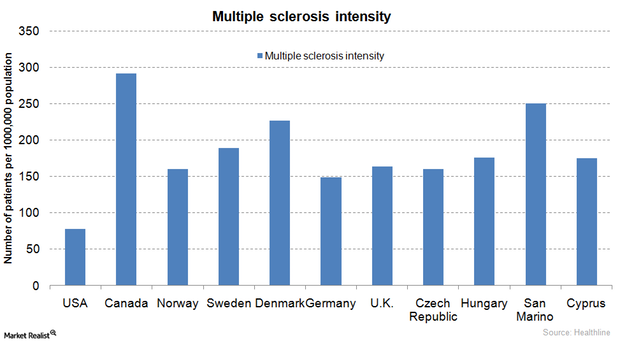
The Biotechnology Industry and Multiple Sclerosis Therapies
Most multiple sclerosis drugs are very costly at about $55,000 per year. The FDA’s April 2015 approval of a generic version of Copaxone is expected to lower the overall price of the therapy.
