Ajovy and Austedo: Teva Pharmaceutical’s Growth Strategy
On April 2, Teva Pharmaceutical issued a press release announcing the European Union’s approval of its monthly and quarterly dosages of Ajovy for preventing migraines.
May 15 2019, Published 4:25 p.m. ET
Ajovy’s market expansion strategy
On April 2, Teva Pharmaceutical (TEVA) issued a press release announcing the European Union’s approval of its monthly and quarterly dosages of Ajovy for preventing migraines.
On its first-quarter earnings conference call, Teva Pharmaceutical highlighted the probability of Ajovy’s earning higher net revenue per patient in the European market than in the US market based on the assumption that the drug’s list price in Europe would be similar to its list price in the United States. This expectation is in line with the trend that we’ve seen for other biopharmaceutical injectables launched in Europe. Additionally, there’s not much difference in the net and list prices of drugs in Europe.
According to Teva’s first-quarter earnings conference call, there’s significant unmet demand for migraine prophylaxis in Europe. To leverage this opportunity, Teva Pharmaceutical plans to launch Ajovy first in markets such as Germany and Scandinavia and later in Southern European markets. The company will, however, need to overcome access constraints for Ajovy in certain markets.
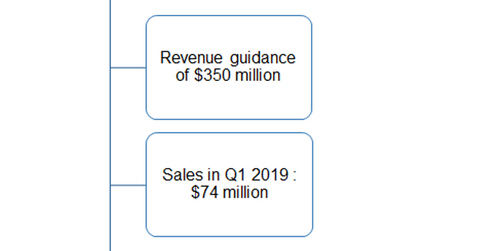
Austedo’s growth trends
On its first-quarter earnings conference call, Teva Pharmaceutical reaffirmed its 2019 annual sales guidance of $350 million for Austedo. The drug reported net sales of $74 million in the first quarter and $208 million in 2018.
According to Teva’s first-quarter earnings investor presentation, more than 18,000 prescriptions of Austedo were dispensed in the first quarter. Around 89% of commercially covered lives and 85% of Medicare Part D covered lives had access to Austedo at the end of the first quarter.
According to the company’s first-quarter earnings conference call, there are ~50,000 tardive dyskinesia patients in the United States. Austedo is competing with Neurocrine Biosciences’ Ingrezza in this segment, which is a highly underserved area.
