Teva’s Key Drugs in Its Specialty Products Pipeline
Teva’s Austedo, approved by the FDA in April 2017, is one of the company’s major growth drivers.
Nov. 20 2020, Updated 11:42 a.m. ET
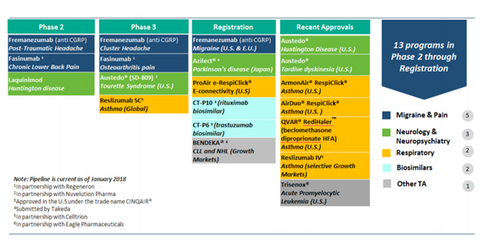
Teva’s specialty product pipeline
Teva Pharmaceutical’s (TEVA) fremanezumab is a key product awaiting FDA approval. It recently received a September PFUDA (Prescription Drug User Fee Act) action date by the FDA for the treatment of migraine.
The drug is also under Phase 2 trials for the treatment of post-traumatic headache and a Phase 3 trial for the treatment of cluster headache. The drug is expected to generate significant sales for the company in the years ahead. Teva’s specialty products pipeline is shown in the chart below.
Teva’s Austedo trials
Teva’s Austedo, approved by the FDA in April 2017, is one of the company’s major growth drivers. Austedo generated sales of $30.0 million in the first quarter, and the company expects the drug to generate sales of $200.0 million in fiscal 2018.
The drug is approved for the treatment of chorea associated with Huntington’s disease, movement disorder related to Huntington’s disease, and tardive dyskinesia. The drug is under a Phase 3 trial for the treatment of Tourette syndrome. For more on the drug, please read Teva’s Austedo Sales Continue to Gain Momentum in 2018.
Other key pipeline drugs
Fainumab is a key pipeline product that’s under a Phase 2 trial for the treatment of chronic lower back pain and under a Phase 3 trial for the treatment of osteoarthritis pain.
Teva’s Bendeka is being developed in partnership with Eagle Pharmaceuticals (EGRX) for the treatment of individuals with chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma. Bendeka is under FDA review.
Eagle Pharmaceuticals recently won a lawsuit against the FDA, which triggered a positive stock reaction for Teva stock. This development increased investor optimism for the approval of Bendeka. The event is expected to boost Teva’s sales. The FDA had previously refused to grant orphan drug status to Bendeka.
Be sure to check out all the data we’ve added to our quote pages. Now you can get a valuation snapshot, earnings and revenue estimates, and historical data, as well as dividend information. Take a look!
