Regeneron Pharmaceuticals’ Kevzara, Arcalyst, and Zaltrap
In January 2017, Regeneron Pharmaceuticals’ (REGN) Kevzara was approved in Canada for the treatment of patients with active rheumatoid arthritis.
Feb. 22 2018, Updated 1:36 p.m. ET
Kevzara solution for subcutaneous injection
In January 2017, Regeneron Pharmaceuticals’ (REGN) Kevzara was approved in Canada for the treatment of patients with active rheumatoid arthritis who are intolerant to disease-modifying anti-rheumatic drugs. That was the first approval of Kevzara worldwide.
The FDA (U.S. Food & Drug Administration) approved Kevzara in May 2017 for the same indication. The product also received marketing authorization from the European Commission in June 2017 in combination with methotrexate for the treatment of rheumatoid arthritis. In September 2017, Kevzara was approved in Japan for the treatment of patients with rheumatoid arthritis.
In fiscal 2017, Kevzara generated sales of $13.3 million. Under a collaboration agreement, Regeneron Pharmaceuticals and Sanofi share profits and losses from the Kevzara sales.
Arcalyst injection
Regeneron Pharmaceuticals’ Arcalyst injection is sold in the United States for the treatment of cryoprin-associated periodic syndromes (or CAPS), familial cold autoinflammatory syndrome (or FCAS), and Muckle-Wells Syndrome (or MWS) in adults and children 12 years or older.
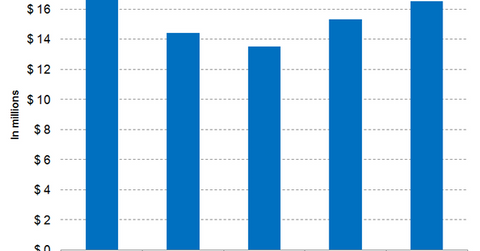
Arcalyst generated revenues of $16.5 million in fiscal 2017 compared with $15.3 million in fiscal 2016.
Zaltrap injection
Zaltrap is marketed in the United States, the European Union, and other countries for the treatment of patients with metastatic colorectal cancer (or mCRC) in combination with leucovorin. Under a 2015 collaboration agreement between Regeneron Pharmaceuticals and Sanofi, the latter is responsible for the development and commercialization of Zaltrap and pays a certain percentage of net sales of the product to the former.
Globally, Zaltrap generated $83.8 million in sales in fiscal 2017 compared with $72.3 million in fiscal 2016.
In the next part of this series, we’ll take a look at Regeneron Pharmaceuticals’ product portfolio.
