Valeant Pharmaceuticals’ Xifaxan and Apriso
In 1H17, Valeant Pharmaceuticals’ (VRX) Xifaxan reported revenues of $418.0 million compared to $408.0 million in 1H16.
Oct. 24 2017, Updated 7:40 a.m. ET
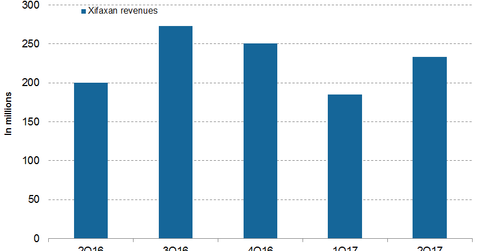
Xifaxan revenue trends
In 1H17, Valeant Pharmaceuticals International’s (VRX) Xifaxan reported revenues of $418.0 million compared to $408.0 million in 1H16. In 2Q17, Xifaxan generated revenues of $233.0 million, which reflected a ~17.0% rise on a YoY (year-over-year) basis and a ~26.0% rise on a QoQ (quarter-over-quarter) basis. In 2Q17, Xifaxan prescriptions rose 2.0% compared to 2Q16.
In 1Q17 and 2Q17, Xifaxan made good progress. High sales in May and June primarily contributed to revenue growth in 2Q17. New prescriptions from the end of 1Q17 to the end of 2Q17 rose from 73.0% to 77.0%. Valeant Pharmaceuticals’ sales team is actively working to increase Xifaxan’s market share.
About Xifaxan
Xifaxan (rifaximin) is an antibacterial used for the treatment of diarrhea caused by noninvasive strains of Escherichia coli. The drug is also used for reducing the risk of overt hepatic encephalopathy recurrence and the treatment of diarrhea patients with irritable bowel syndrome.
Apriso revenue trends
In 1H17, Apriso reported revenues of $68.0 million compared to $65.0 million in 1H16. In 2Q17, Apriso generated revenues of $39.0 million, which reflected a ~22.0% rise YoY and a ~34.0% rise QoQ. Apriso is used for the treatment of adult individuals with moderate ulcerative colitis. In 2Q17 Apriso prescriptions rose 7.0% compared to 2Q16.
Growth in sales for Valeant Pharmaceuticals’ Xifaxan could boost the stock of the Global X Guru Index ETF (GURU). Valeant Pharmaceuticals makes up ~0.20% of GURU’s total portfolio holdings.
