BioMarin’s Strong Pipeline Could Be a Long-Term Growth Driver
After success in the company’s phase 1/2 trial with BMN 270, an investigational gene therapy for hemophilia A, BioMarin Pharmaceuticals (BMRN) is expected to start phase 3 trials.
Sept. 12 2017, Updated 7:37 a.m. ET
Clinical trials with BMN 270
After success in the company’s phase 1/2 trial with BMN 270, an investigational gene therapy for hemophilia A, BioMarin Pharmaceuticals (BMRN) is expected to start phase 3 trials. In August 2017, BioMarin Pharmaceuticals announced its plans to start two separate phase 3 trials for 4e13 vg/kg dose and 6e13vg/kg dose.
In the phase 1/2 trial in November and December, at week 32, all three patients who were administered 4e13 vg/kg dose achieved a normal range of factor VIII activity levels, demonstrating median and mean factor VIII levels of 51%. Also, among the three patients who were administered 4e13 vg/kg dose in February and March 2017, everyone’s Factor VIII activity levels had progressed to a mild range. Two of the three patients continued to show an upward trend of factor VIII activity levels.
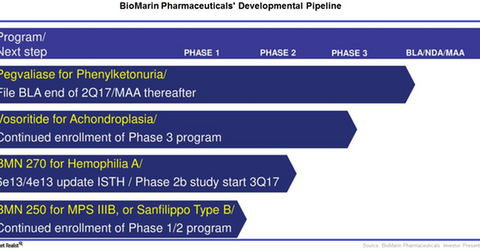
The above chart shows BioMarin Pharmaceuticals’ pipeline portfolio.
Clinical trial with BMN 250
In September 2017, BioMarin Pharmaceuticals presented interim statistics from the dose escalation arm of phase 1/2 trial with BMN 250. BMN 250 is an enzyme replacement therapy under investigation that utilizes a novel fusion of recombinant human Alpha-N-acetylglucosaminidase (or NAGLU) with a derived peptide of insulin-like growth factor 2 (or IGF2) for the treatment of individuals with Sanfilippo B syndrome or mucopolysaccharidosis IIIB (or MPS IIIB). The phase 1/2 trial demonstrated tolerability of ICV-administered BMN 250 in Sanfilippo B patients. The primary aim of the study was to evaluate the safety of BMN 250 in Sanfilippo B patients.
BioMarin Pharmaceuticals’ peers in the hemophilia drug market include Pfizer (PFE), Bioverativ (BIVV), Bayer (BAYZF), Novo Nordisk (NVO), CSL, and others. The iShares Russell Mid-Cap Growth ETF (IWP) invests ~0.55% of its total portfolio holdings in BioMarin Pharmaceuticals.
