Stryker Stock Falls Due to the Impact of the Sage Products Recall
On August 23, Stryker (SYK) announced a voluntary product recall of specific lots of oral care products that form part of the company’s Sage business unit.
Aug. 31 2017, Published 2:32 p.m. ET
Sage products recall
On August 23, Stryker (SYK) announced a voluntary product recall of specific lots of oral care products that form part of the company’s Sage business unit. The recall came in response to a warning letter Stryker receive from the FDA (US Food and Drug Administration) on July 17. Stryker’s stock price fell around 4% following the news of the recall.
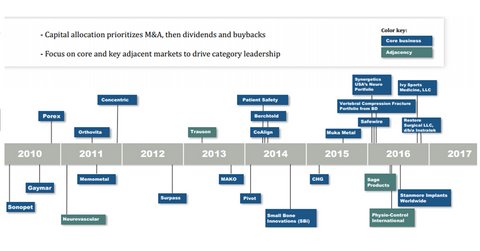
As the above acquisition timeline shows, Stryker had acquired Sage Products in April 2016 as part of its expansion plans across key adjacent markets. The business became part of Stryker’s MedSurg product portfolio. The MedSurg division recorded strong performance in 2Q17.
The products that were recalled comprised oral care solutions manufactured by a third-party supplier for Stryker’s Sage Products unit. These products were distributed between July 2015 and August 2017.
Major competitors Medtronic (MDT), Zimmer Biomet Holdings (ZBH), and Johnson & Johnson (JNJ) have also seen some major recalls over recent years. Investors can consider investing in the iShares Core S&P 500 ETF (IVV) for exposure to Stryker and, at the same time, diversifying company- and industry-specific risks. IVV invests ~0.21% of its total holdings in SYK.
Why did Stryker recall Sage’s oral care products?
In the warning letter, the FDA raised cross-contamination concerns for oral care solutions manufactured by the third party. The solutions were also made on the same equipment as other non-pharmaceutical products. As per Stryker, there haven’t been any adverse events related to these products. However, some cases of minor irritation and allergic reactions were reported.
The FDA has also raised concerns about the “microbiological testing methods used for all products containing solutions sold by Sage.” The products mentioned by the FDA include recalled Oral Care solutions and Sage-manufactured cloth-based products. Th FDA has mandated the testing of these products using a verified compendial microbiological method that can determine the type and number of microorganisms as well in addition to their detection. Sage was previously using a less time-consuming testing method that could detect the presence of microorganisms.
Consequently, Stryker has suspended the shipments of its cloth-based products until the testing concludes. These products constitute 50% of Sage sales. As per Stryker, it’s expected to resume shipments in September and return to full capacity supply by the end of fiscal 2017.
Also, Stryker updated its full fiscal 2017 guidance as a result of the event. Let’s discuss the updated guidance in detail in the next part of this series.
